Abstract
AIM—To study changes induced in ocular surface epithelia and the tear film by antiglaucomatous eyedrops. A β blocker (0.5% timolol) and a novel prostaglandin F2α metabolite related drug (0.12% unoprostone) were examined in a prospective, randomised fashion. METHODS—40 patients were randomly assigned to use either 0.5% timolol (timolol group) or 0.12% unoprostone eyedrops (unoprostone group) twice a day for 24 weeks. In addition to routine ocular examinations, corneal epithelial integrity (vital staining tests, tear film break up time (BUT), anterior fluorometry, specular microscopy) and tear function (Schirmer's test, cotton thread test, tear clearance test (TCT)) were examined before and after the treatment. RESULTS—Both eyedrops caused significant reduction in intraocular pressure from the baseline levels. No significant changes were noted in corneal integrity in both groups, except a decrease in BUT at 20 weeks in the timolol group. The timolol group demonstrated significant decreases in Schirmer's test, tear clearance test, and tear function index (Schirmer's test value multiplied by clearance test); however, no such changes were noted in the unoprostone group. CONCLUSION—While unoprostone eyedrops caused no adverse effects on the corneal epithelial integrity and tear function, timolol caused significant impairments in tear production and turnover.
Full Text
The Full Text of this article is available as a PDF (151.2 KB).
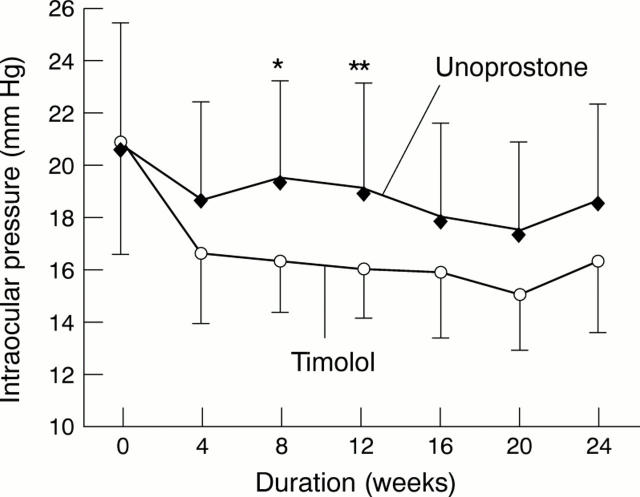
Figure 1 .
Changes in intraocular pressure in the unoprostone and timolol groups. *p=0.0086, **p=0.014 between the unoprostone and timolol groups.
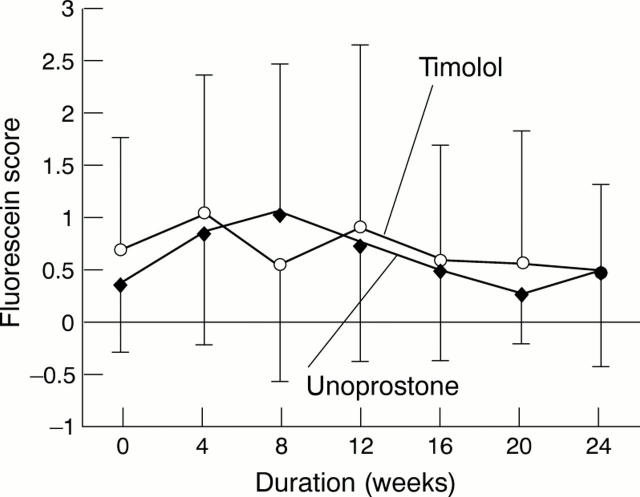
Figure 2 .
Changes in fluorescein scores in the timolol and unoprostone groups.
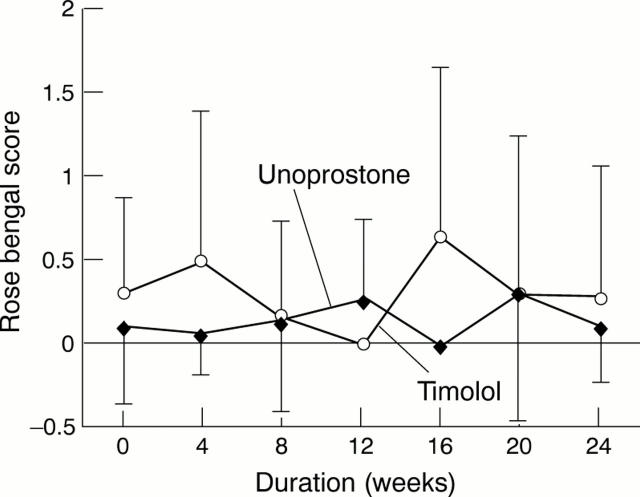
Figure 3 .
Changes in rose bengal scores in the timolol and unoprostone groups.
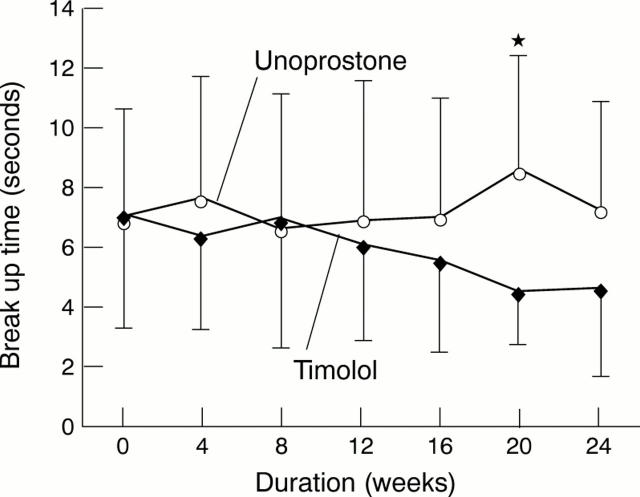
Figure 4 .
Changes in BUT in the unoprostone and timolol groups. *p = 0.0087 between the timolol and unoprostone groups.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma I., Masuda K., Kitazawa Y., Takase M., Yamamura H. Double-masked comparative study of UF-021 and timolol ophthalmic solutions in patients with primary open-angle glaucoma or ocular hypertension. Jpn J Ophthalmol. 1993;37(4):514–525. [PubMed] [Google Scholar]
- Camras C. B. Mechanism of the prostaglandin-induced reduction of intraocular pressure in humans. Adv Prostaglandin Thromboxane Leukot Res. 1995;23:519–525. [PubMed] [Google Scholar]
- Haria M., Spencer C. M. Unoprostone (isopropyl unoprostone) Drugs Aging. 1996 Sep;9(3):213–220. doi: 10.2165/00002512-199609030-00007. [DOI] [PubMed] [Google Scholar]
- Herreras J. M., Pastor J. C., Calonge M., Asensio V. M. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology. 1992 Jul;99(7):1082–1088. doi: 10.1016/s0161-6420(92)31847-0. [DOI] [PubMed] [Google Scholar]
- Mishima H. K., Kiuchi Y., Takamatsu M., Rácz P., Bito L. Z. Circadian intraocular pressure management with latanoprost: diurnal and nocturnal intraocular pressure reduction and increased uveoscleral outflow. Surv Ophthalmol. 1997 Feb;41 (Suppl 2):S139–S144. doi: 10.1016/s0039-6257(97)80021-5. [DOI] [PubMed] [Google Scholar]
- Reidy J. J., Zarzour J., Thompson H. W., Beuerman R. W. Effect of topical beta blockers on corneal epithelial wound healing in the rabbit. Br J Ophthalmol. 1994 May;78(5):377–380. doi: 10.1136/bjo.78.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto R., Bennett E. S., Henry V. A., Paragina S., Narumi T., Izumi Y., Kamei Y., Nagatomi E., Miyanaga Y., Hamano H. The phenol red thread tear test: a cross-cultural study. Invest Ophthalmol Vis Sci. 1993 Dec;34(13):3510–3514. [PubMed] [Google Scholar]
- Sakurai M., Araie M., Oshika T., Mori M., Shoji N., Masuda K. Effects of topical application of UF-021, a novel prostaglandin-related compound, on aqueous humor dynamics in rabbit. Jpn J Ophthalmol. 1993;37(3):252–258. [PubMed] [Google Scholar]
- Sherwood M. B., Grierson I., Millar L., Hitchings R. A. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon's capsule in glaucomatous patients. Ophthalmology. 1989 Mar;96(3):327–335. doi: 10.1016/s0161-6420(89)32888-0. [DOI] [PubMed] [Google Scholar]
- Shimazaki J., Fujishima H., Yagi Y., Tsubota K. Effects of diclofenac eye drops on corneal epithelial structure and function after small-incision cataract surgery. Ophthalmology. 1996 Jan;103(1):50–57. doi: 10.1016/s0161-6420(96)30732-x. [DOI] [PubMed] [Google Scholar]
- Toda I., Tsubota K. Practical double vital staining for ocular surface evaluation. Cornea. 1993 Jul;12(4):366–367. doi: 10.1097/00003226-199307000-00015. [DOI] [PubMed] [Google Scholar]
- Toris C. B., Camras C. B., Yablonski M. E. Effects of PhXA41, a new prostaglandin F2 alpha analog, on aqueous humor dynamics in human eyes. Ophthalmology. 1993 Sep;100(9):1297–1304. doi: 10.1016/s0161-6420(93)31484-3. [DOI] [PubMed] [Google Scholar]
- Toshino A., Okamoto S., Shimamura I., Miyamoto F., Hara Y., Kodama T., Ohashi Y. [The mechanism of corneal epithelial disorder induced by prostaglandin F2 alpha isopropyl unoprostone]. Nippon Ganka Gakkai Zasshi. 1998 Feb;102(2):101–105. [PubMed] [Google Scholar]
- Trope G. E., Liu G. S., Basu P. K. Toxic effects of topically administered Betagan, Betoptic, and Timoptic on regenerating corneal epithelium. J Ocul Pharmacol. 1988 Winter;4(4):359–366. doi: 10.1089/jop.1988.4.359. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Chiba K., Shimazaki J. Corneal epithelium in diabetic patients. Cornea. 1991 Mar;10(2):156–160. doi: 10.1097/00003226-199103000-00011. [DOI] [PubMed] [Google Scholar]
- Tsubota K. In vivo observation of the corneal epithelium. Scanning. 1994 Sep-Oct;16(5):295–299. [PubMed] [Google Scholar]
- Tsubota K., Yamada M. Corneal epithelial alterations induced by disposable contact lens wear. Ophthalmology. 1992 Aug;99(8):1193–1196. doi: 10.1016/s0161-6420(92)31824-x. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Yamada M., Naoi S. Specular microscopic observation of human corneal epithelial abnormalities. Ophthalmology. 1991 Feb;98(2):184–191. doi: 10.1016/s0161-6420(91)32318-2. [DOI] [PubMed] [Google Scholar]
- Van Buskirk E. M. Adverse reactions from timolol administration. Ophthalmology. 1980 May;87(5):447–450. doi: 10.1016/s0161-6420(80)35215-9. [DOI] [PubMed] [Google Scholar]
- Weissman S. S., Asbell P. A. Effects of topical timolol (0.5%) and betaxolol (0.5%) on corneal sensitivity. Br J Ophthalmol. 1990 Jul;74(7):409–412. doi: 10.1136/bjo.74.7.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. M., 2nd Adverse external ocular effects of topical ophthalmic medications. Surv Ophthalmol. 1979 Sep-Oct;24(2):57–88. doi: 10.1016/0039-6257(79)90125-5. [DOI] [PubMed] [Google Scholar]
- Xu K. P., Yagi Y., Toda I., Tsubota K. Tear function index. A new measure of dry eye. Arch Ophthalmol. 1995 Jan;113(1):84–88. doi: 10.1001/archopht.1995.01100010086025. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Kitazawa Y., Azuma I., Masuda K. Clinical evaluation of UF-021 (Rescula; isopropyl unoprostone). Surv Ophthalmol. 1997 Feb;41 (Suppl 2):S99–103. doi: 10.1016/s0039-6257(97)80015-x. [DOI] [PubMed] [Google Scholar]
- Yokoi N., Kinoshita S. Clinical evaluation of corneal epithelial barrier function with the slit-lamp fluorophotometer. Cornea. 1995 Sep;14(5):485–489. [PubMed] [Google Scholar]
- de Jong C., Stolwijk T., Kuppens E., de Keizer R., van Best J. Topical timolol with and without benzalkonium chloride: epithelial permeability and autofluorescence of the cornea in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1994 Apr;232(4):221–224. doi: 10.1007/BF00184009. [DOI] [PubMed] [Google Scholar]
- van Bijsterveld O. P. Diagnostic tests in the Sicca syndrome. Arch Ophthalmol. 1969 Jul;82(1):10–14. doi: 10.1001/archopht.1969.00990020012003. [DOI] [PubMed] [Google Scholar]