Abstract
AIM—To investigate the APO*E3-Leiden mouse as an animal model for age related maculopathy (ARM) related extracellular deposits. METHODS—Eyes were obtained from APO*E3-Leiden transgenic mice on a high fat/cholesterol (HFC) diet (n=12) or on a normal mouse chow (n=6), for 9 months. As controls, eyes were collected from APO-E knockout mice on the same diets. From each mouse one eye was processed for microscopic evaluation and immunohistochemistry with a polyclonal antibody directed against human apo-E. Electron microscopy was also performed. RESULTS—All 12 eyes of the APO*E3-Leiden mice on an HFC diet contained basal laminar deposit (BLD; class 1 to class 3), whereas two of six APO*E3-Leiden mice on normal chow showed BLD class 1. The ultrastructural aspects of this BLD were comparable with those seen in early BLD in humans, and BLD showed immunoreaction with anti-human-apo-E antibodies. No BLD was found in any of the control mice. Drusen were not detected in any of the mice. CONCLUSION—These results indicate that APO*E3-Leiden mice can be used as animal model for the pathogenesis of BLD, and that a HFC diet enhances the accumulation of this deposit. Furthermore, this study supports the previously suggested involvement of dysfunctional apo-E in the accumulation of extracellular deposits in ARM.
Full Text
The Full Text of this article is available as a PDF (224.1 KB).
Figure 1 .
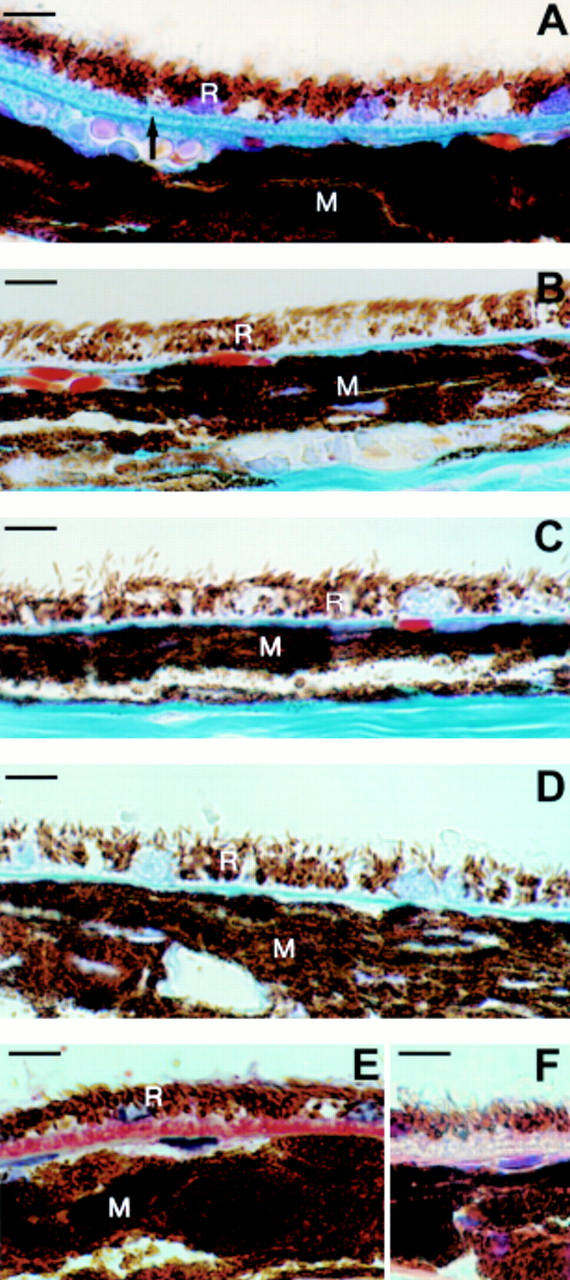
Light microscopic images of (A) a continuous layer (class 2) of basal laminar deposit (arrow), between the retinal pigment epithelium (R) and Bruch's membrane, in the central retina of an APO*E3-Leiden mouse on a high fat/cholesterol (HFC) diet. (B) the central retina from an APO*E3-Leiden mouse on normal chow, without basal laminar deposit (BLD class 0). Note the thin Bruch's membrane between the RPE (R) and the choroidal melanin (M). (C, D) The central retinas of APO-E knockout mice (without BLD) on a (C) normal, and (D) HFC diet, respectively. (E, F) a continuous layer (class 2) of basal laminar deposit, between the RPE and Bruch's membrane, in the central retina of an APO*E3-Leiden mouse on an HFC diet. (E) Stained with an anti-human apo-e antibody (BLD stains red). (F) Negative control. Scale bar indicates 10 µm.
Figure 2 .

Electron micrograph of basal laminar deposit (BLD) class 2, between the cell membrane of the retinal pigment epithelium (RPE) and the basement membrane of the RPE, in a central retina from an APO*E3-Leiden mouse on a high fat/cholesterol diet. Furthermore, there is an increase and elongation of the basal infoldings of the RPE (*). Uranyl acetate/lead citrate staining. Scale bar indicates 1.1 µm. N = nucleus of RPE; C = choriocapillaris.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. C., Bressler N. M., Bressler S. B., Chisholm I. H., Coscas G., Davis M. D., de Jong P. T., Klaver C. C., Klein B. E., Klein R. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995 Mar-Apr;39(5):367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- Bird A. C. Bruch's membrane change with age. Br J Ophthalmol. 1992 Mar;76(3):166–168. doi: 10.1136/bjo.76.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles J. K., Zoellner C. D., Anderson L. J., Kosik L. M., Pitas R. E., Weisgraber K. H., Hui D. Y., Mahley R. W., Gebicke-Haerter P. J., Ignatius M. J. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest. 1989 Mar;83(3):1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow J. L. Mouse models of atherosclerosis. Science. 1996 May 3;272(5262):685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- Green W. R., Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993 Oct;100(10):1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Green W. R., McDonnell P. J., Yeo J. H. Pathologic features of senile macular degeneration. Ophthalmology. 1985 May;92(5):615–627. [PubMed] [Google Scholar]
- Havekes L., de Wit E., Leuven J. G., Klasen E., Utermann G., Weber W., Beisiegel U. Apolipoprotein E3-Leiden. A new variant of human apolipoprotein E associated with familial type III hyperlipoproteinemia. Hum Genet. 1986 Jun;73(2):157–163. doi: 10.1007/BF00291607. [DOI] [PubMed] [Google Scholar]
- Holz F. G., Sheraidah G., Pauleikhoff D., Bird A. C. Analysis of lipid deposits extracted from human macular and peripheral Bruch's membrane. Arch Ophthalmol. 1994 Mar;112(3):402–406. doi: 10.1001/archopht.1994.01090150132035. [DOI] [PubMed] [Google Scholar]
- Klaver C. C., Kliffen M., van Duijn C. M., Hofman A., Cruts M., Grobbee D. E., van Broeckhoven C., de Jong P. T. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998 Jul;63(1):200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver C. C., Wolfs R. C., Vingerling J. R., Hofman A., de Jong P. T. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998 May;116(5):653–658. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B. E., Linton K. L. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992 Jun;99(6):933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- Kliffen M., Mooy C. M., Luider T. M., Huijmans J. G., Kerkvliet S., de Jong P. T. Identification of glycosaminoglycans in age-related macular deposits. Arch Ophthalmol. 1996 Aug;114(8):1009–1014. doi: 10.1001/archopht.1996.01100140217021. [DOI] [PubMed] [Google Scholar]
- Kliffen M., Mooy C. M., Luider T. M., de Jong P. T. Analysis of carbohydrate structures in basal laminar deposit in aging human maculae. Invest Ophthalmol Vis Sci. 1994 Jun;35(7):2901–2905. [PubMed] [Google Scholar]
- Kliffen M., de Jong P. T., Luider T. M. Protein analysis of human maculae in relation to age-related maculopathy. Lab Invest. 1995 Aug;73(2):267–272. [PubMed] [Google Scholar]
- Kliffen M., van der Schaft T. L., Mooy C. M., de Jong P. T. Morphologic changes in age-related maculopathy. Microsc Res Tech. 1997 Jan 15;36(2):106–122. doi: 10.1002/(SICI)1097-0029(19970115)36:2<106::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Lutgens E., Daemen M., Kockx M., Doevendans P., Hofker M., Havekes L., Wellens H., de Muinck E. D. Atherosclerosis in APOE*3-Leiden transgenic mice: from proliferative to atheromatous stage. Circulation. 1999 Jan 19;99(2):276–283. doi: 10.1161/01.cir.99.2.276. [DOI] [PubMed] [Google Scholar]
- Löffler K. U., Lee W. R. Basal linear deposit in the human macula. Graefes Arch Clin Exp Ophthalmol. 1986;224(6):493–501. doi: 10.1007/BF02154735. [DOI] [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Miceli M. V., Newsome D. A., Tate D. J., Jr, Sarphie T. G. Pathologic changes in the retinal pigment epithelium and Bruch's membrane of fat-fed atherogenic mice. Curr Eye Res. 2000 Jan;20(1):8–16. [PubMed] [Google Scholar]
- Moore D. J., Hussain A. A., Marshall J. Age-related variation in the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci. 1995 Jun;36(7):1290–1297. [PubMed] [Google Scholar]
- Mullins R. F., Johnson L. V., Anderson D. H., Hageman G. S. Characterization of drusen-associated glycoconjugates. Ophthalmology. 1997 Feb;104(2):288–294. doi: 10.1016/s0161-6420(97)30322-4. [DOI] [PubMed] [Google Scholar]
- Mullins R. F., Russell S. R., Anderson D. H., Hageman G. S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000 May;14(7):835–846. [PubMed] [Google Scholar]
- Pauleikhoff D., Barondes M. J., Minassian D., Chisholm I. H., Bird A. C. Drusen as risk factors in age-related macular disease. Am J Ophthalmol. 1990 Jan 15;109(1):38–43. doi: 10.1016/s0002-9394(14)75576-x. [DOI] [PubMed] [Google Scholar]
- Pauleikhoff D., Zuels S., Sheraidah G. S., Marshall J., Wessing A., Bird A. C. Correlation between biochemical composition and fluorescein binding of deposits in Bruch's membrane. Ophthalmology. 1992 Oct;99(10):1548–1553. doi: 10.1016/s0161-6420(92)31768-3. [DOI] [PubMed] [Google Scholar]
- Sarks S. H. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976 May;60(5):324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger S. A., Iwahashi C. K., Hjelmeland L. M., Davis P. A., Morse L. S. Apolipoprotein E in the subretinal fluid of rhegmatogenous and exudative retinal detachments. Retina. 1997;17(1):38–43. doi: 10.1097/00006982-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Sheraidah G., Steinmetz R., Maguire J., Pauleikhoff D., Marshall J., Bird A. C. Correlation between lipids extracted from Bruch's membrane and age. Ophthalmology. 1993 Jan;100(1):47–51. doi: 10.1016/s0161-6420(13)31712-6. [DOI] [PubMed] [Google Scholar]
- Starita C., Hussain A. A., Marshall J. Decreasing hydraulic conductivity of Bruch's membrane: relevance to photoreceptor survival and lipofuscinoses. Am J Med Genet. 1995 Jun 5;57(2):235–237. doi: 10.1002/ajmg.1320570224. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Roses A. D. Apolipoprotein E and Alzheimer disease. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4725–4727. doi: 10.1073/pnas.92.11.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerling J. R., Dielemans I., Bots M. L., Hofman A., Grobbee D. E., de Jong P. T. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam Study. Am J Epidemiol. 1995 Aug 15;142(4):404–409. doi: 10.1093/oxfordjournals.aje.a117648. [DOI] [PubMed] [Google Scholar]
- Wilson C., Wardell M. R., Weisgraber K. H., Mahley R. W., Agard D. A. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science. 1991 Jun 28;252(5014):1817–1822. doi: 10.1126/science.2063194. [DOI] [PubMed] [Google Scholar]
- van Vlijmen B. J., van den Maagdenberg A. M., Gijbels M. J., van der Boom H., HogenEsch H., Frants R. R., Hofker M. H., Havekes L. M. Diet-induced hyperlipoproteinemia and atherosclerosis in apolipoprotein E3-Leiden transgenic mice. J Clin Invest. 1994 Apr;93(4):1403–1410. doi: 10.1172/JCI117117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Maagdenberg A. M., Hofker M. H., Krimpenfort P. J., de Bruijn I., van Vlijmen B., van der Boom H., Havekes L. M., Frants R. R. Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia. J Biol Chem. 1993 May 15;268(14):10540–10545. [PubMed] [Google Scholar]
- van der Schaft T. L., Mooy C. M., de Bruijn W. C., Bosman F. T., de Jong P. T. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol. 1994 Jan;232(1):40–46. doi: 10.1007/BF00176436. [DOI] [PubMed] [Google Scholar]
- van der Schaft T. L., Mooy C. M., de Bruijn W. C., Oron F. G., Mulder P. G., de Jong P. T. Histologic features of the early stages of age-related macular degeneration. A statistical analysis. Ophthalmology. 1992 Feb;99(2):278–286. doi: 10.1016/s0161-6420(92)31982-7. [DOI] [PubMed] [Google Scholar]
- van der Schaft T. L., de Bruijn W. C., Mooy C. M., Ketelaars D. A., de Jong P. T. Is basal laminar deposit unique for age-related macular degeneration? Arch Ophthalmol. 1991 Mar;109(3):420–425. doi: 10.1001/archopht.1991.01080030122052. [DOI] [PubMed] [Google Scholar]
- van der Schaft T. L., de Bruijn W. C., Mooy C. M., de Jong P. T. Basal laminar deposit in the aging peripheral human retina. Graefes Arch Clin Exp Ophthalmol. 1993 Aug;231(8):470–475. doi: 10.1007/BF02044234. [DOI] [PubMed] [Google Scholar]


