Abstract
BACKGROUND/AIMS—Fluid transport across the in vitro corneal epithelium is short lived, hence difficult to detect and characterise. Since stable rates of fluid transport across several cultured epithelial cell layers have been demonstrated, the behaviour of confluent SV40 transformed rabbit corneal epithelial cells (tRCEC) grown on permeable supports was examined. METHODS—Fluid transport was determined with a nanoinjector volume clamp; the specific electrical resistance of the layers was 184 (SEM 9) Ω cm2. tRCEC layers transported fluid (from basal to apical) against a pressure head of 3 cm H2O for 2-3 hours. RESULTS—In the first hour, the rate of fluid transport was 5.2 (0.5) µl/h/cm−2 (n=23), which is comparable with that found in other epithelia. Fluid transport was completely inhibited in 15-30 minutes by either 100 µM ouabain (n=6), 50 µM bumetanide (n=6), or 1 µM endothelin-1 (ET-1; n=6). Preincubation with 10 µM BQ123 (an ETA receptor antagonist) obviated inhibition by ET-1 (n=6). ET-1 also caused a 22% decrease in specific resistance. CONCLUSIONS—Fluid transport appears to depend on transepithelial Cl transport since (1) their directions are the same (stroma→tear), and (2) both bumetanide and ouabain inhibit it with similar time course. tRCEC appear useful to investigate aspects of the physiology and pharmacology of fluid transport across this layer, including receptor mediated control of this process.
Full Text
The Full Text of this article is available as a PDF (203.5 KB).
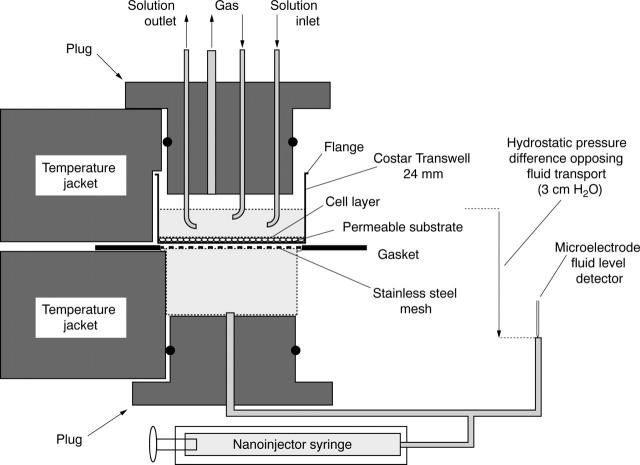
Figure 1 .
Schematic cross sectional diagram of the experimental setup used to measure fluid transport across epithelial cell layers (tRCEC). The right half of the chamber has been omitted so as to provide labels for the various elements within the chamber compartments.
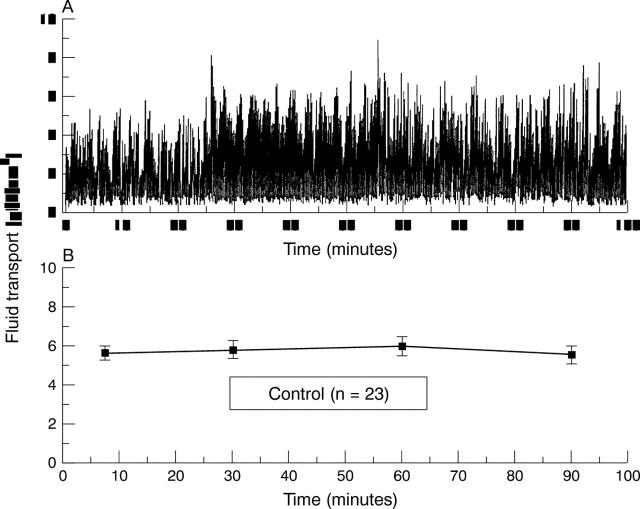
Figure 2 .
(A) An individual recording of fluid transport. Upward deflections correspond to fluid moving in the basolateral to apical direction, from the bottom to the upper chamber. Each deflection represents the amount of fluid transported in 12 seconds. (B) Average fluid flow recorded from 23 experiments.
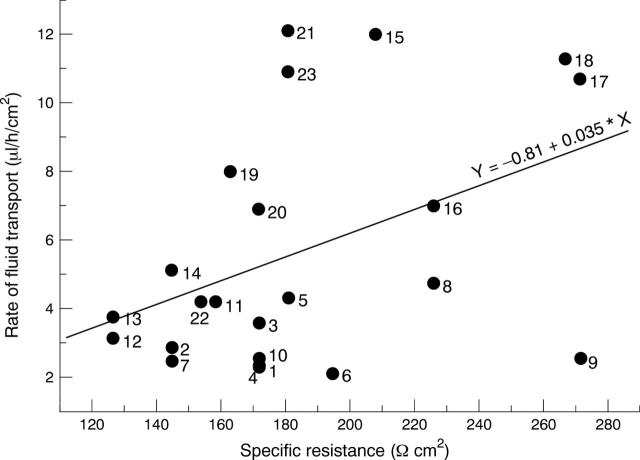
Figure 3 .
Relation between the rate of fluid transport and electrical resistance of the 23 cultured layers referred to in Figure 2B. Each point denotes the maximal rate for a given experiment, averaged for a period of 30 minutes; labels beside symbols denote the experiment number. A regression line fitted to the data is shown, along with its variables.
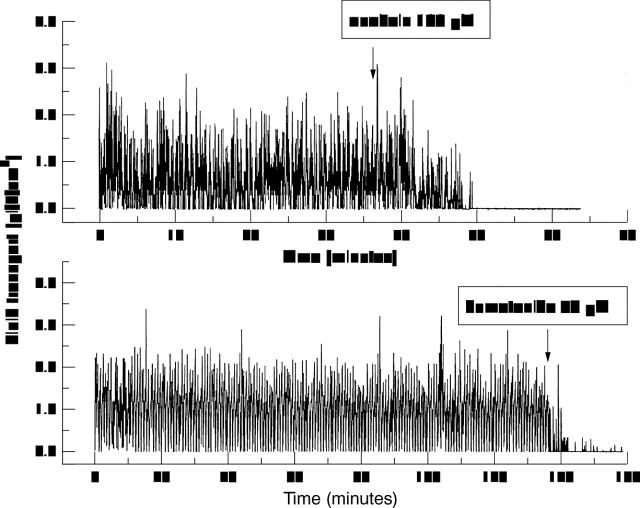
Figure 4 .
Recording of fluid transport as in Figure 2. Top: a representative experiment (n=6), showing the inhibitory effect of 100 µM ouabain added to the apical side of a cell layer. Bottom: similarly, a representative experiment (n=6) showing the inhibitory effect of 50 µM bumetanide ouabain added to the apical side.
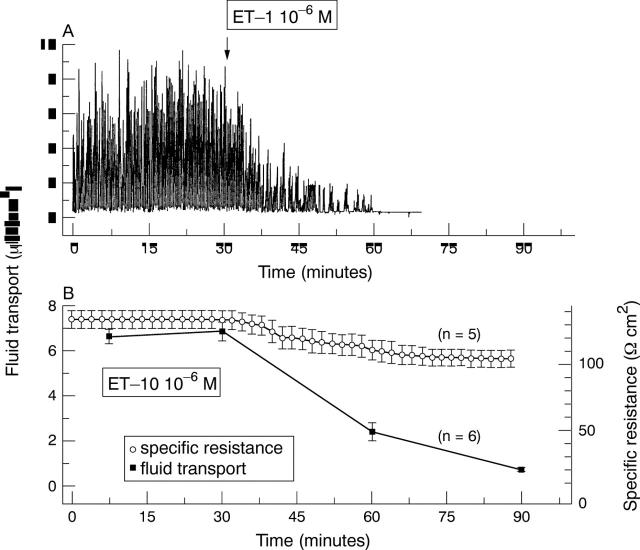
Figure 5 .
(A) An individual recording shows the inhibitory effect of ET-1 on fluid transport. ET-1 (final concentration: 1 µM) was added to the apical side of the cells. (B) Effects of ET-1 on average rates of fluid flow (n= 6). This panel also shows the average specific electrical resistance of the layers before and after addition of 1 µM ET-1.
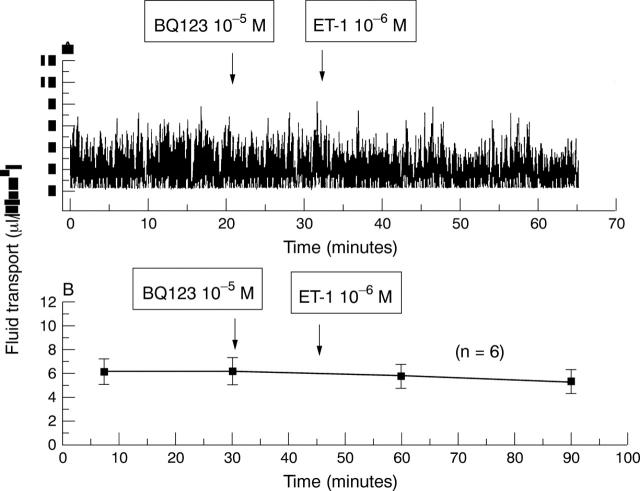
Figure 6 .
(A) BQ123 added to the apical side (final concentration: 10 µM) forestalls the inhibitory effect of 1 µM ET-1. (B) Effects of BQ123 (n=6).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOURGUET J., JARD S. UN DISPOSITIF AUTOMATIQUE DE MESURE ET D'ENREGISTREMENT DU FLUX NET D'EAU 'A TRAVERS LA PEAU ET LA VESSIE DES AMPHIBIENS. Biochim Biophys Acta. 1964 Sep 25;88:442–444. [PubMed] [Google Scholar]
- Bonanno J. A., Klyce S. D., Cragoe E. J., Jr Mechanism of chloride uptake in rabbit corneal epithelium. Am J Physiol. 1989 Aug;257(2 Pt 1):C290–C296. doi: 10.1152/ajpcell.1989.257.2.C290. [DOI] [PubMed] [Google Scholar]
- Candia O. A., Zamudio A. C. Chloride-activated water permeability in the frog corneal epithelium. J Membr Biol. 1995 Feb;143(3):259–266. doi: 10.1007/BF00233454. [DOI] [PubMed] [Google Scholar]
- Cohen S. R., Polse K. A., Brand R. J., Mandell R. B. Humidity effects on corneal hydration. Invest Ophthalmol Vis Sci. 1990 Jul;31(7):1282–1287. [PubMed] [Google Scholar]
- Echevarría M., Kuang K., Iserovich P., Li J., Preston G. M., Agre P., Fischbarg J. Cultured bovine corneal endothelial cells express CHIP28 water channels. Am J Physiol. 1993 Nov;265(5 Pt 1):C1349–C1355. doi: 10.1152/ajpcell.1993.265.5.C1349. [DOI] [PubMed] [Google Scholar]
- Fischbarg J., Lim J. J., Bourguet J. Adenosine stimulation of fluid transport across rabbit corneal endothelium. J Membr Biol. 1977 Jun 30;35(2):95–112. doi: 10.1007/BF01869942. [DOI] [PubMed] [Google Scholar]
- Hakvoort A., Haselbach M., Galla H. J. Active transport properties of porcine choroid plexus cells in culture. Brain Res. 1998 Jun 8;795(1-2):247–256. doi: 10.1016/s0006-8993(98)00284-4. [DOI] [PubMed] [Google Scholar]
- Horwich T., Ibarra C., Ford P., Zamudio A., Parisi M., Candia O. A. mRNA from frog corneal epithelium increases water permeability in Xenopus oocytes. Invest Ophthalmol Vis Sci. 1995 Dec;36(13):2772–2774. [PubMed] [Google Scholar]
- Kang F., Kuang K., Li J., Fischbarg J. Cultured bovine corneal epithelial cells express a functional aquaporin water channel. Invest Ophthalmol Vis Sci. 1999 Jan;40(1):253–257. [PubMed] [Google Scholar]
- Klyce S. D. Enhancing fluid secretion by the corneal epithelium. Invest Ophthalmol Vis Sci. 1977 Oct;16(10):968–973. [PubMed] [Google Scholar]
- Klyce S. D. Transport of Na, Cl, and water by the rabbit corneal epithelium at resting potential. Am J Physiol. 1975 May;228(5):1446–1452. doi: 10.1152/ajplegacy.1975.228.5.1446. [DOI] [PubMed] [Google Scholar]
- Klyce S. D., Wong R. K. Site and mode of adrenaline action on chloride transport across the rabbit corneal epithelium. J Physiol. 1977 Apr;266(3):777–799. doi: 10.1113/jphysiol.1977.sp011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice D. M. The location of the fluid pump in the cornea. J Physiol. 1972 Feb;221(1):43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima S., Hedbys B. O. The permeability of the corneal epithelium and endothelium to water. Exp Eye Res. 1967 Jan;6(1):10–32. doi: 10.1016/s0014-4835(67)80049-6. [DOI] [PubMed] [Google Scholar]
- Narula P., Xu M., Kuang K. Y., Akiyama R., Fischbarg J. Fluid transport across cultured bovine corneal endothelial cell monolayers. Am J Physiol. 1992 Jan;262(1 Pt 1):C98–103. doi: 10.1152/ajpcell.1992.262.1.C98. [DOI] [PubMed] [Google Scholar]
- O'Neal M. R., Polse K. A. In vivo assessment of mechanisms controlling corneal hydration. Invest Ophthalmol Vis Sci. 1985 Jun;26(6):849–856. [PubMed] [Google Scholar]
- Oishi R., Nonoguchi H., Tomita K., Marumo F. Endothelin-1 inhibits AVP-stimulated osmotic water permeability in rat inner medullary collecting duct. Am J Physiol. 1991 Dec;261(6 Pt 2):F951–F956. doi: 10.1152/ajprenal.1991.261.6.F951. [DOI] [PubMed] [Google Scholar]
- Raina S., Preston G. M., Guggino W. B., Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995 Jan 27;270(4):1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- Takagi H., Reinach P. S., Tachado S. D., Yoshimura N. Endothelin-mediated cell signaling and proliferation in cultured rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):134–142. [PubMed] [Google Scholar]
- Takagi H., Reinach P. S., Yoshimura N., Honda Y. Endothelin-1 promotes corneal epithelial wound healing in rabbits. Curr Eye Res. 1994 Aug;13(8):625–628. doi: 10.3109/02713689408999897. [DOI] [PubMed] [Google Scholar]
- Takashima Y., Takagi H., Takahashi M., Reinach P. S., Mircheff A. K., Warren D. W., Yoshimura N. Endothelin protein expression in tear glands of the rabbit. Curr Eye Res. 1996 Jul;15(7):768–773. doi: 10.3109/02713689609003461. [DOI] [PubMed] [Google Scholar]
- Tao W., Wu X., Liou G. I., Abney T. O., Reinach P. S. Endothelin receptor-mediated Ca2+ signaling and isoform expression in bovine corneal epithelial cells. Invest Ophthalmol Vis Sci. 1997 Jan;38(1):130–141. [PubMed] [Google Scholar]
- Tomita K., Nonoguchi H., Terada Y., Marumo F. Effects of ET-1 on water and chloride transport in cortical collecting ducts of the rat. Am J Physiol. 1993 Apr;264(4 Pt 2):F690–F696. doi: 10.1152/ajprenal.1993.264.4.F690. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Jr, Jones K. L., Alves K., Chan C. P., Hollis G. F., Tung J. S. Characterization of cloned human endothelin receptors. Life Sci. 1993;53(5):407–414. doi: 10.1016/0024-3205(93)90644-i. [DOI] [PubMed] [Google Scholar]
- Zadunaisky J. A. Active transport of chloride in frog cornea. Am J Physiol. 1966 Aug;211(2):506–512. doi: 10.1152/ajplegacy.1966.211.2.506. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Kuang K., Kang F., Li J., Fischbarg J. Platelet activating factor inhibits fluid transport by corneal endothelium. Invest Ophthalmol Vis Sci. 1996 Aug;37(9):1899–1906. [PubMed] [Google Scholar]