Abstract
AIMS—To examine the hypothesis that apoptosis of infiltrating cells contributes to spontaneous resolution of uveitis in clinically relevant rodent models. METHODS—Experimental melanin induced uveitis (EMIU) was induced in Fischer 344 rats by immunisation with 250 µg bovine ocular melanin. Endotoxin induced uveitis (EIU) was induced by injection of 200 µg Escherichia coli lipopolysaccharide. Formalin fixed, paraffin embedded ocular cross sections were stained by terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate biotin nick end labelling (TUNEL) to identify apoptotic cells. Indirect immunoperoxidase staining of paraformaldehyde lysine periodate fixed tissue cross sections was used to demonstrate expression of inducible nitric oxide synthase (iNOS). RESULTS—TUNEL positive mononuclear cells were observed in the anterior uvea during both EMIU and EIU at all selected time points. However, whereas the majority of mononuclear cells appeared apoptotic from the outset of disease, neutrophils were notably TUNEL negative at all time points examined. Many infiltrating neutrophils expressed iNOS. CONCLUSION—Apoptosis occurs early in the course of rat EMIU and EIU, and may contribute to resolution of these diseases. In general, infiltrating mononuclear cells die rapidly, while neutrophils survive, producing inducible nitric oxide synthase which may contribute to disease pathogenesis.
Full Text
The Full Text of this article is available as a PDF (254.7 KB).
Figure 1 .
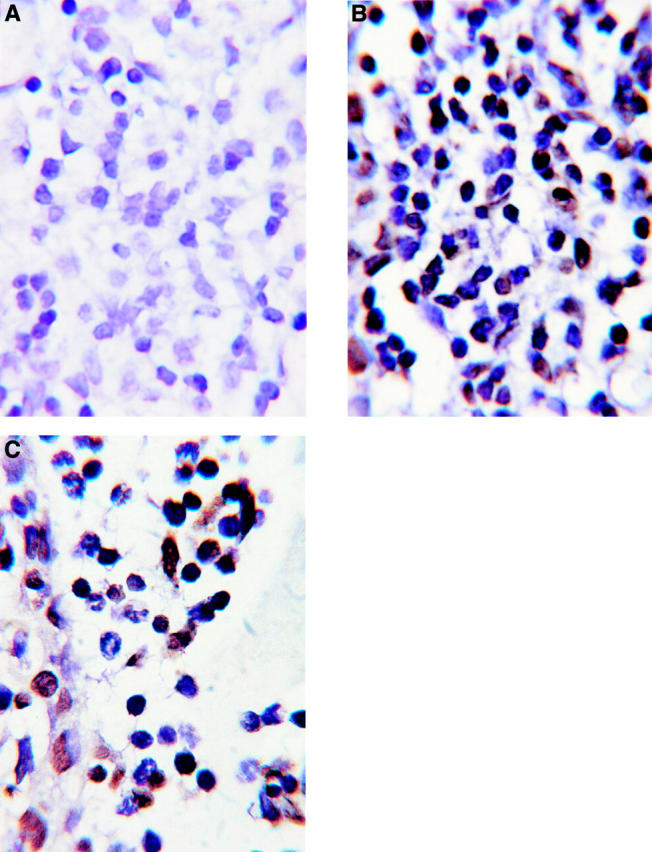
Sections of a Fischer 344 rat eye during early EMIU (at time of disease onset), stained by TUNEL. (A) Ciliary body, negative control: no biotinylated dUTP. (B) Ciliary body, test, showing many TUNEL positive (apoptotic) mononuclear cells. (C) Anterior chamber, test, showing TUNEL positive mononuclear cells and TUNEL negative neutrophils. Haematoxylin counterstain. Magnification ×1400.
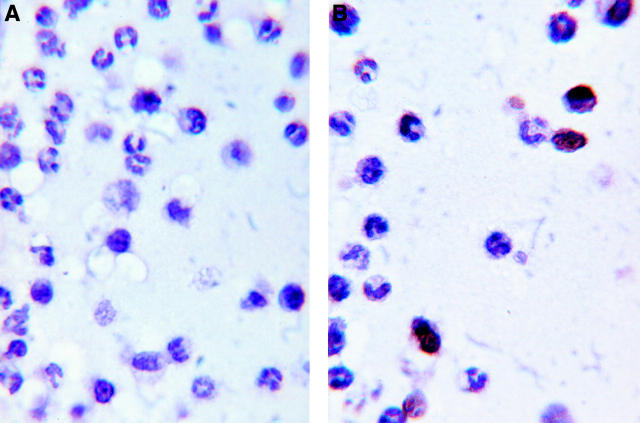
Figure 2 .
Photomicrograph demonstrating TUNEL positive mononuclear cells and TUNEL negative polymorphonuclear cells in the aqueous exudate of a Fischer 344 rat with EIU, 24 hours after lipopolysaccharide injection. (A) Negative control: no biotinylated dUTP; (B) TUNEL test run. Haematoxylin counterstain. Magnification ×1400.
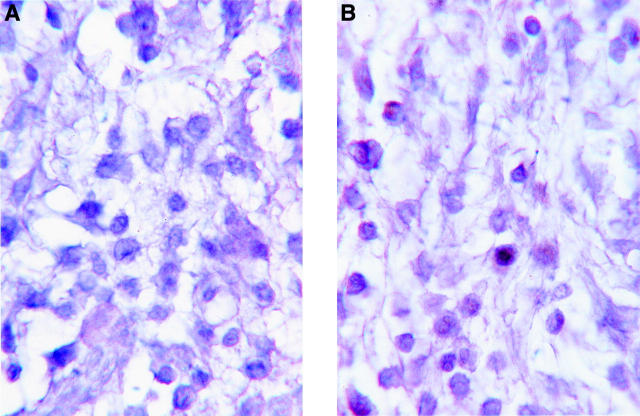
Figure 3 .
Photomicrograph of an inflamed ankle joint from a DA rat with adjuvant arthritis, showing very few TUNEL positive infiltrating cells. (A) Negative control: no biotinylated dUTP; (B) TUNEL test run. Haematoxylin counterstain. Magnification ×1400.
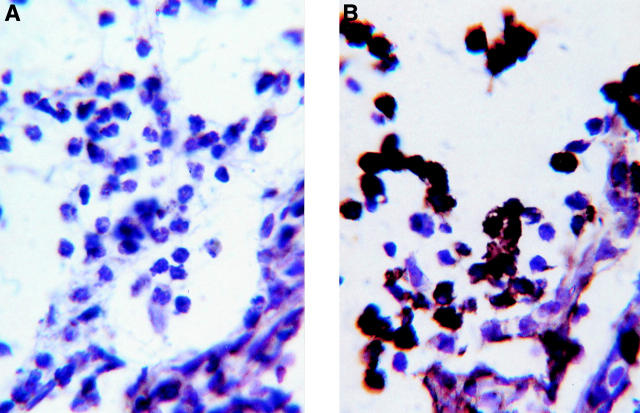
Figure 4 .
Photomicrograph demonstrating iNOS in cells in the anterior uvea and aqueous exudate of a F344 rat at the onset of EMIU. (A) Negative control: SAL5; (B) anti-iNOS antibody. Immunoperoxidase stain with haematoxylin counterstain. Magnification ×1400.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broekhuyse R. M., Kuhlmann E. D. Experimental autoimmune anterior uveitis. The preparation of uveitogenic ocular melanin. Invest Ophthalmol Vis Sci. 1993 Mar;34(3):698–700. [PubMed] [Google Scholar]
- Broekhuyse R. M., Kuhlmann E. D., Winkens H. J. Experimental autoimmune anterior uveitis (EAAU). III. Induction by immunization with purified uveal and skin melanins. Exp Eye Res. 1993 May;56(5):575–583. doi: 10.1006/exer.1993.1071. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Matteson D. M., Li Q., Whitcup S. M., Nussenblatt R. B. Apoptosis in patients with posterior uveitis. Arch Ophthalmol. 1997 Dec;115(12):1559–1567. doi: 10.1001/archopht.1997.01100160729010. [DOI] [PubMed] [Google Scholar]
- De Vos A. F., Klaren V. N., Kijlstra A. Simultaneous induction of cytokine mRNA expression in the anterior uvea during endotoxin-induced uveitis in the rat. Exp Eye Res. 1996 May;62(5):581–584. doi: 10.1006/exer.1996.0068. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goureau O., Bellot J., Thillaye B., Courtois Y., de Kozak Y. Increased nitric oxide production in endotoxin-induced uveitis. Reduction of uveitis by an inhibitor of nitric oxide synthase. J Immunol. 1995 Jun 15;154(12):6518–6523. [PubMed] [Google Scholar]
- Griffith T. S., Brunner T., Fletcher S. M., Green D. R., Ferguson T. A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995 Nov 17;270(5239):1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- Griffith T. S., Ferguson T. A. The role of FasL-induced apoptosis in immune privilege. Immunol Today. 1997 May;18(5):240–244. doi: 10.1016/s0167-5699(97)81663-5. [DOI] [PubMed] [Google Scholar]
- Jacquemin E., de Kozak Y., Thillaye B., Courtois Y., Goureau O. Expression of inducible nitric oxide synthase in the eye from endotoxin-induced uveitis rats. Invest Ophthalmol Vis Sci. 1996 May;37(6):1187–1196. [PubMed] [Google Scholar]
- Kogiso M., Tanouchi Y., Mimura Y., Nagasawa H., Himeno K. Endotoxin-induced uveitis in mice. 1. Induction of uveitis and role of T lymphocytes. Jpn J Ophthalmol. 1992;36(3):281–290. [PubMed] [Google Scholar]
- Lowenstein C. J., Dinerman J. L., Snyder S. H. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994 Feb 1;120(3):227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- Magnusson C., Vaux D. L. Signalling by CD95 and TNF receptors: not only life and death. Immunol Cell Biol. 1999 Feb;77(1):41–46. doi: 10.1046/j.1440-1711.1999.00800.x. [DOI] [PubMed] [Google Scholar]
- Mandai M., Mittag T. W., Kogishi J., Iwaki M., Hangai M., Yoshimura N. Role of nitric oxide synthase isozymes in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1996 Apr;37(5):826–832. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- McMenamin P. G., Crewe J. M. Cellular localisation and dynamics of nitric oxide synthase expression in the rat anterior segment during endotoxin-induced uveitis. Exp Eye Res. 1997 Aug;65(2):157–164. doi: 10.1006/exer.1997.0323. [DOI] [PubMed] [Google Scholar]
- McMenamin P. G., Crewe J. Endotoxin-induced uveitis. Kinetics and phenotype of the inflammatory cell infiltrate and the response of the resident tissue macrophages and dendritic cells in the iris and ciliary body. Invest Ophthalmol Vis Sci. 1995 Sep;36(10):1949–1959. [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Parks D. J., Cheung M. K., Chan C. C., Roberge F. G. The role of nitric oxide in uveitis. Arch Ophthalmol. 1994 Apr;112(4):544–546. doi: 10.1001/archopht.1994.01090160124032. [DOI] [PubMed] [Google Scholar]
- Planck S. R., Huang X. N., Robertson J. E., Rosenbaum J. T. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):924–930. [PubMed] [Google Scholar]
- Rosenbaum J. T., McDevitt H. O., Guss R. B., Egbert P. R. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980 Aug 7;286(5773):611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- Rothova A., van Veenedaal W. G., Linssen A., Glasius E., Kijlstra A., de Jong P. T. Clinical features of acute anterior uveitis. Am J Ophthalmol. 1987 Feb 15;103(2):137–145. doi: 10.1016/s0002-9394(14)74218-7. [DOI] [PubMed] [Google Scholar]
- Smith J. R., Hart P. H., Coster D. J., Williams K. A. Mice deficient in tumor necrosis factor receptors p55 and p75, interleukin-4, or inducible nitric oxide synthase are susceptible to endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1998 Mar;39(3):658–661. [PubMed] [Google Scholar]
- Smith J. R., Hart P. H., Parish C. R., Standfield S. D., Coster D. J., Williams K. A. Experimental melanin-induced uveitis in the Fischer 344 rat is inhibited by anti-CD4 monoclonal antibody, but not by mannose-6-phosphate. Clin Exp Immunol. 1999 Jan;115(1):64–71. doi: 10.1046/j.1365-2249.1999.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spargo L. D., Hawkes J. S., Cleland L. G., Mayrhofer G. Recruitment of lymphoblasts derived from peripheral and intestinal lymph to synovium and other tissues in normal rats and rats with adjuvant arthritis. J Immunol. 1996 Dec 1;157(11):5198–5207. [PubMed] [Google Scholar]
- Stuart P. M., Griffith T. S., Usui N., Pepose J., Yu X., Ferguson T. A. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997 Feb 1;99(3):396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. G., Chang K., Corbett J. A., Misko T. P., Currie M. G., Bora N. S., Kaplan H. J., Williamson J. R. Endotoxin-induced uveitis in the rat is attenuated by inhibition of nitric oxide production. Invest Ophthalmol Vis Sci. 1994 Jul;35(8):3278–3288. [PubMed] [Google Scholar]
- Watson R. W., Rotstein O. D., Jimenez M., Parodo J., Marshall J. C. Augmented intracellular glutathione inhibits Fas-triggered apoptosis of activated human neutrophils. Blood. 1997 Jun 1;89(11):4175–4181. [PubMed] [Google Scholar]
- Woon M. D., Kaplan H. J., Bora N. S. Kinetics of cytokine production in experimental autoimmune anterior uveitis (EAAU). Curr Eye Res. 1998 Oct;17(10):955–961. doi: 10.1076/ceyr.17.10.955.5246. [DOI] [PubMed] [Google Scholar]
- Yamagami S., Kawashima H., Tsuru T., Yamagami H., Kayagaki N., Yagita H., Okumura K., Gregerson D. S. Role of Fas-Fas ligand interactions in the immunorejection of allogeneic mouse corneal transplants. Transplantation. 1997 Oct 27;64(8):1107–1111. doi: 10.1097/00007890-199710270-00004. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Yoshimura N., Hangai M., Tanihara H., Honda Y. Interleukin-1 alpha, interleukin-1 beta, and tumor necrosis factor gene expression in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):1107–1113. [PubMed] [Google Scholar]
- de Vos A. F., Klaren V. N., Kijlstra A. Expression of multiple cytokines and IL-1RA in the uvea and retina during endotoxin-induced uveitis in the rat. Invest Ophthalmol Vis Sci. 1994 Oct;35(11):3873–3883. [PubMed] [Google Scholar]