Abstract
Glial cells support the survival and development of central neurons through the supply of trophic factors. Here we demonstrate that l-serine (l-Ser) and glycine (Gly) also are glia-derived trophic factors. These amino acids are released by astroglial cells and promote the survival, dendritogenesis, and electrophysiological development of cultured cerebellar Purkinje neurons. Although l-Ser and Gly are generally classified as nonessential amino acids, 3-phosphoglycerate dehydrogenase (3PGDH), a key enzyme for their biosynthesis, is not expressed in Purkinje neurons. By contrast, the Bergman glia, a native astroglia in the cerebellar cortex, highly expresses 3PGDH. These data suggest that l-Ser and Gly mediate the trophic actions of glial cells on Purkinje neurons.
The survival and development of neurons in the central nervous system (CNS) critically depend on various trophic factors supplied by surrounding neurons and glial cells (1). Purkinje neurons (PN), the sole efferent elements in the cerebellar cortex, provide a suitable model for investigating such neuron–neuron and neuron–glia trophic interactions. The structural simplicity of the cerebellar cortex has facilitated the characterization of cellular interactions influencing the postnatal development of PN (2–4). Cerebellar granule neurons are suggested to regulate the survival and dendritic differentiation of PN during the postnatal period (4, 5). Recent studies have shown that a balance between glutamatergic and brain-derived neurotrophic factor (BDNF) signalings from granule neurons is required for the normal survival and dendritic development of PN (6). Glial cells are also suggested to have trophic actions on PN. In cultures with or without granule neurons, the survival and neurite growth of PN are enhanced by media conditioned by astroglia (7, 8) or glial cell line-derived neurotrophic factor (9). Among glial cell types in the cerebellum, the Bergmann glia is thought to be most influential on PN because of its intimate spatial association with PN (2, 10). Phenotypes of vimentin-null mutant mice support this notion. The lack of vimentin, an intermediate filament protein, abolishes the intimate association of the Bergmann glia with PN and consequently leads to the necrotic death of PN (11). However, the molecular nature of factors mediating Bergmann glia's trophic actions on PN remains unknown.
Here we demonstrate that the nonessential amino acids, l-Ser and Gly have strong trophic actions on PN in vitro. These amino acids are identified as the major active components of cultured astroglia-derived trophic factors for PN. The Bergmann glia appears to be the main source of these amino acids to PN in vivo, because, in the cerebellar cortex, only these cells express the mRNA and protein of 3-phosphoglycerate dehydrogenase (3PGDH), a key enzyme in the biosynthesis of l-Ser and Gly (12, 13). These findings reveal a previously unrecognized role of the simple amino acids as mediators of the astroglial trophic actions on CNS neurons.
Materials and Methods
Primary Cultures and Conditioned Media.
Cerebellar neuronal cultures were prepared from Wistar rat fetuses (embryonic day 20–21) and maintained in 12-well culture plates (Falcon 3043) under glia- and serum-free conditions (14). The serum-free medium was composed of minimum essential medium (MEM, GIBCO no. 61100) supplemented with bovine insulin (20 μg/ml), BSA (100 μg/ml), cytosine arabinonucleoside (2 μM), gentamicin (10 μg/ml), l-glutamine (200 μg/ml; final concentration in the medium: 3.37 mM), Hepes (25 mM), human apotransferrin (200 μg/ml), progesterone (40 nM), putrescine (100 μM), pyruvate (500 μM), sodium selenite (30 nM), and triiodothyronine (0.5 ng/ml). Cerebellar granule cell cultures were prepared from postnatal day 4 rat pups and maintained on 6-well culture plates (6 × 105 cells per well) as described above. On day in vitro (DIV) 4, half of the medium was replaced with fresh medium. The medium was collected on DIV7 and used as conditioned medium from granule neurons. Primary cerebellar and cerebral astroglial cultures were prepared from day 21 embryos and day 4 pups, respectively, by a published method (15) and maintained in MEM supplemented with gentamicin (10 μg/ml), l-glutamine (200 μg/ml; final concentration in the medium: 3.37 mM), Hepes (25 mM), and 10% FBS until reaching confluence (16). Then, astroglial cells were fed with the serum-free MEM with supplements listed above except cytosine arabinonucleoside. The serum-free medium was replaced with fresh medium every 3 days and the media recovered from the 2nd to 4th changes were examined and used as medium conditioned by astroglial cells. When preparing conditioned medium, the ratios of cells to medium were kept to 6.0 × 105 cells per ml for granule neurons and 3.0–4.0 × 105 cells per ml for astroglial cultures. All pharmacological manipulations were done on DIV0, and cell counting and morphological evaluation were done on DIV12–14, except where noted. Each treatment was performed at least in duplicate. To determine the density of surviving PN, we photomicrographed randomly chosen 1-mm2 areas of the cell layer, and counted PN immunostained for calbindin D-28K in each photomicrograph. In some experiments, we counted labeled PN under a phase-contrast microscope. For each treatment, fields corresponding to 16–32% of the area of the cell layer were measured. Experimental controls were taken from each 12-well culture plate (2 wells per plate). Statistical analysis was performed by prism (version 2.0b, GraphPad Software).
Reconstitution Experiment.
A medium conditioned by cerebellar astroglia cells (CeACM) was separated into two fractions by a centrifugal size-exclusion filter (Centriplus YM-3, cut-off molecular weight 3,000; Millipore). After this manipulation, the concentrations of l-Ser and Gly in the macromolecular fraction with molecular weights of >3,000 were decreased to 5.6 ± 1.4 μM and 6.3 ± 0.8 μM (n = 3), respectively. The low weight fraction with molecular weights of <3,000 retained all amino acids found in CeACM. When these two fractions were added together to cultures, PN survival was improved to the level comparable to that in CeACM treatment (Fig. 1F, column 1). CeACM was reconstituted by the serial additions of the macromolecular fraction, MEM amino acid mixture composed of l-isomers of Arg, Cys, His, Ile, Lys, Met, Phe, Thr, Trp, Tyr, and Val (GIBCO/BRL no. 11130), and l-Ser (100 μM)/Gly (40 μM) mix to an Earle's balanced salt solution (GIBCO/BRL no. 24010) supplemented with MEM vitamin mixture (GIBCO/BRL no. 11120), l-glutamine (3.37 mM), Hepes (25 mM), putrescine (100 μM), and pyruvate (500 μM). Effect of treatment with a reconstituted CeACM (50% vol/vol) on PN survival was examined as described above.
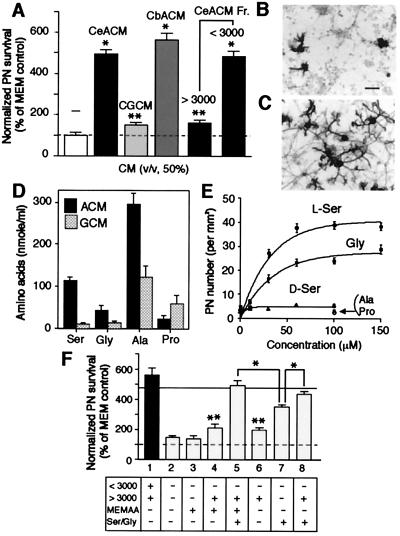
Figure 1.
Identification of l-Ser and Gly as major PN survival-promoting factors released by cerebellar astroglial cells. Cerebellar neurons (2.0 × 105 cells per well) were maintained in the serum free-MEM medium (final volume: 0.4 ml per well) for 14 days in the presence of CeACM (50%, vol/vol), its fraction, CbACM, or CGCM. On DIV14, cultures of PN were stained for calbindin D-28K for counting and morphological evaluation. (A) PN viability was expressed as normalized value (% of PN numbers per mm2 in sister controls, the mean ± SEM of two or three independent experiments, n = 60–72). Statistical significance versus MEM control (Student's unpaired t test): *, P < 0.0001; **, P < 0.02. (B and C) Phenotypic appearance of PN in 14 day culture in the absence of CeACM (B) or in the presence of CeACM (C). (Bar, 50 μm.) (D) Amino acids released in CeACM or CGCM were analyzed with an L-8500 amino acid analyzer (Hitachi). In addition to these amino acids, we detected taurine (6.34 μM), l-Asp (0.81 μM), and l-Glu (5.91 μM) in CeACM. (E) Effect of amino acid treatment on PN survival was examined as in A. l-Ser (●), Gly (■), d-Ser (▴), l-Ala (▾), l-Pro (○). (F) Effect of reconstituted CeACM treatment was examined as in A. Results were expressed as normalized value as in A (the mean ± SEM of two independent experiments, n = 60). The composition of each reconstituted CeACM is shown. MEMAA indicates MEM amino acid mixture. The line indicates the level of PN survival of cultures treated with CeACM. Statistical significance (Student's unpaired t test): *, P < 0.0001 (versus column 5, 8, or CeACM treatment); **, P < 0.001 (versus MEM control). Note that no significant difference was detected between CeACM treatment and column 5 or 8.
Electrophysiology.
Whole-cell recordings were performed in a ruptured- or perforated-patch configuration at 26°C (series resistance for 48 PN, 60.4 ± 3.8 MΩ) (17). Briefly, the pipette solution for the perforated-patch recordings consisted of (mM): 100 d-gluconic acid potassium salt, 10 NaOH, 3.3 CaCl2, 4 MgCl2, 10 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrapotassium salt, and 10 Hepes (pH, 7.5); amphotericin B (200 μg/ml) or gramicidin D (500 μg/ml) (18) was added before use. The pipette solution for the ruptured-patch recordings had the same cations and contained 138 mM Cl− and 2 mM ATP. The bath solution was MEM supplemented with 5 mM Hepes and 500 μg/ml BSA (pH, 7.2). Signals were amplified, filtered at 2–5 kHz, and sampled at 10 kHz by using an Axopatch-1D amplifier (Axon) and PULSE 8.10 system (HEKA Electronics, Lambrecht, Germany). Cerebellar granule neurons were identified by their soma size (diameter, 6–7 μm) (19), and PN were identified by their soma size (diameter, ≥15 μm) and/or by immunostaining for calbindin D-28K. Command and measured potentials were corrected for a liquid junction potential between the solutions.
In Situ Hybridization and Immunohistochemistry.
Antisense and sense oligonucleotides against two nonoverlapping coding sequences of the rat 3PGDH mRNA, nucleotide residues 94–138 and 1537–1581 (GenBank accession no. X97772; ref. 20), were synthesized and labeled with deoxyadenosine 5′-[α-[35S]thio] triphosphate (0.5 × 109 dpm/μg of DNA). Frozen sections of adult Wistar rat brain (20 μm) were processed for hybridization (21). Polyclonal antibodies against 3PGDH were raised by immunizing rabbits with purified rat 3PGDH protein genetically expressed in Escherichia coli. The specificity for 3PGDH was confirmed by Western blot analysis. Brain sections (50 μm) were incubated with anti-3PGDH antibodies (0.2 μg/ml) and mouse anti-glial fibrillary acidic protein (GFAP) antibody (1 μg/ml, Boehringer Mannheim). Immunoreaction was visualized with Cy3-labeled anti-rabbit IgG or FITC-labeled anti-mouse IgG (1:200; Jackson ImmunoResearch). For preembedding immunoelectron microscopy, microslicer sections immunoreacted with the antibody were incubated with biotinylated anti-rabbit secondary antibody and then with streptavidin-peroxidase. They were further incubated with 3,3′-diaminobenzidine as a peroxidase substrate. Following postfixation with osmium tetroxide and block staining with uranyl acetate, sections were embedded in Epon 812. From the straight portion of lobules 4 + 5 (culmen), silver-gold ultrathin sections were prepared on an ultramicrotome (Leica). Electron micrographs were taken on a Hitachi H7100 electron microscope.
Chemicals.
Amino acids and recombinant neurotrophins were obtained from Nacalai Tesque and Peprotech, respectively. Mouse tumor necrosis factor α (TNFα) and human tumor necrosis factor β1 (TGFβ1) were from Roche Diagnostics. (S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [(S)-AMPA], d-2-amino-5-phosphonovaleric acid (d-AP5), MK801, 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX), and N-methyl-D-aspartate (NMDA) were from Tocris. Strychnine and tetrodotoxin were from Sigma. All other reagents were analytical grade or equivalent.
Results and Discussion
Cerebellar Astroglia Releases l-Ser and Gly with Major Trophic Activities on PN.
Rat embryo (day 20–21) cerebella were prepared to make the mixed neuronal cultures in serum- and glial cell-free conditions (15) used in all of the present experiments. PN were positively identified by immunostaining for calbindin D-28K (5–6, 14). Without any additional treatment, PN in this culture system poorly survived and differentiated poorly despite the presence of granule neurons (Fig. 1 A and B). Addition of CeACM on DIV0 greatly enhanced both the survival and dendritic differentiation of PN (Fig. 1 A and C) as previously reported (7, 8), whereas addition of a medium conditioned by cerebellar granule neuron-enriched cultures (CGCM) produced a subtle increase in PN survival. In contrast to a previous report (8), a medium conditioned by cerebral (CbACM) or hippocampal astroglial cells also supported PN survival (Fig. 1A and data not shown). Unlike PN, the survival of granule neurons was not improved by the addition of CeACM (data not shown). Heat treatment of the CeACM did not abolish its survival-promoting activity on PN (data not shown). A fractionation study revealed that the components of the CeACM with molecular weights of <3,000 were much more effective than those of >3000 (Fig. 1A, Right). Therefore, the most active components of the CeACM would be small compounds. It was reported that l-Glu, l-Asp, and their related agonists enhance the survival of PN, possibly through the activation of glutamate receptors (22–24) and that l-Ser supports the development of peripheral neurons (25) and hippocampal neurons (16). We therefore tested whether the amino acids contained in the CeACM have trophic effects on PN.
The CeACM obtained by a 3-day incubation with cerebellar astroglial cells was found to contain: l-Ala (296.3 ± 26.0 μM), l-Ser (112.7 ± 9.2 μM), Gly (42.7 ± 10.6 μM), l-Pro (23.1 ± 8.0 μM) (mean ± SEM, n = 6) (Fig. 1D). Although the release of these nonessential amino acids was detectable in CGCM, l-Ser and Gly levels were extremely lower than those in the CeACM. In CbACM, the concentrations for l-Ala and l-Ser were 433.2 ± 42.5 μM and 206.2 ± 14.4 μM, respectively. In an experiment illustrated in Fig. 1E, l-Ser and Gly clearly improved the survival of PN in a dose-dependent and saturable manner. Neither l-Ala nor l-Pro had trophic effect at any concentrations examined. The effect of l-Ser was stereospecific, because d-Ser did not support PN survival.
To estimate the contribution of l-Ser and Gly in the PN survival-promoting activity of CeACM, we prepared partially reconstituted CeACM by adding back of pure amino acids to the macromolecular fraction with molecular weights of >3,000 in a physiological salt solution containing vitamins (see Materials and Methods) and examined their survival-promoting activities. As suggested in Fig. 1A, a reconstituted CeACM that contained the macromolecular fraction with MEM amino acid mixture devoid of l-Ser and Gly had a slight but statistically significant trophic effect on PN (Fig. 1F, column 4), while the addition of l-Ser (100 μM)/Gly (40 μM) to this produced a greater improvement of PN survival comparable to that in the CeACM-treated cultures (column 5). The addition of l-Ser/Gly alone to the physiological salt solution also considerably improved PN survival (column 7). It should be noted, however, that the level of PN survival in cultures treated only with l-Ser and Gly did not reach to the levels of that treated with a mixture of the macromolecular fraction and l-Ser/Gly (compare columns 5 and 8 with 7). A factor in the macromolecular fraction was not a requisite for PN survival, but acted cooperatively with l-Ser/Gly (columns 6–8).
Because it is difficult at present to identify a factor in the macromolecular fraction, we tested whether known protein neurotrophic or neuroprotective factors act synergistically with l-Ser. Among molecules tested, neurotrophin-3 (NT-3) (40 ng/ml) or TNFα (1, 10 ng/ml) showed an additive effect on the l-Ser treatment (Fig. 2A). Although exogenously added NT-3 (26) or TNFα alone improved PN survival in the present culture system (data not shown), their trophic effects were limited and not comparable to l-Ser and Gly (data not shown). Such synergistic enhancement of l-Ser's effect was not seen for either BDNF or TGFβ1, each of which was not trophic on PN (data not shown).
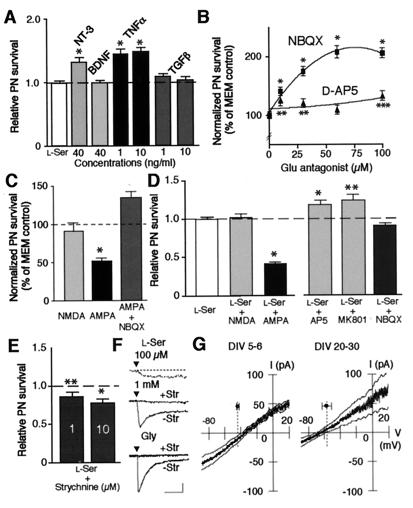
Figure 2.
Characterization of effect of l-Ser and Gly on cerebellar neurons. (A) Synergistic effect of neurotrophin or cytokine treatment with l-Ser (100 μM) was examined as in Fig. 1A. Results were expressed as relative changes to PN numbers per mm2 in cultures treated with 100 μM l-Ser (the mean ± SEM of two independent experiments, n = 50). The line indicates the level of PN survival of cultures treated with 100 μM l-Ser. Statistical significance versus 100 μM l-Ser treatment (Student's unpaired t test): *, P < 0.0001. (B) Effect of Glu receptor antagonist treatment (10, 30, 60 μM) on PN survival was examined on DIV7. PN viability was expressed as normalized value (% of PN numbers per mm2 in controls, the mean ± SEM of two independent experiments, n = 60–72). Statistical significance versus MEM control (Student's unpaired t test): *, P < 0.0001; **, P < 0.05; ***, P < 0.002. (C) Effect of Glu agonist (10 μM) with or without NBQX (30 μM) treatment on PN survival was examined on DIV7 as in B (the mean ± SEM of three independent experiments, n = 72). Statistical significance versus MEM control (Student's unpaired t test): *, P < 0.0001. (D) Effect of Glu agonist (10 μM) or antagonist (30 μM) treatment on the l-Ser trophic action was examined on DIV7 as in A. Results were expressed as relative changes to PN numbers/mm2 in cultures treated with 100 μM l-Ser (the mean ± SEM of two or three independent experiments, n = 48–72). Statistical significance versus 100 μM l-Ser treatment (Student's unpaired t test): *, P < 0.0001; **, P < 0.005. (E) Effect of strychnine treatment on the l-Ser trophic action was examined on DIV7 as in A. Results were expressed as relative changes to PN numbers/mm2 in cultures treated with 100 μM l-Ser (the mean ± SEM of two or three independent experiments, n = 48–72). Statistical significance versus 100 μM l-Ser treatment (Student's unpaired t test): *, P < 0.0001; **, P < 0.01. (F) Currents induced by l-Ser or Gly (100 μM) with (+Str) or without (−Str) strychnine (10 μM). Drug application starts at triangle. Membrane potential held at −60 mV in ruptured-patch mode. Calibration bars: 10 sec and 5, 20, and 100 pA for top, middle, and bottom traces, respectively. (G) The I–V relation of Gly-induced current in granule neurons on indicated DIV. Gly-induced current was extracted as a difference between voltage ramp (−26 mV/sec)-evoked responses recorded in gramicidin-perforated-patch mode before and during application of Gly (100 μM). Mean line is flanked by smoothed ±SEM lines. Dots and error bars: mean and ±SEM of the Erest of the granule neurons. n = 7 for each plot.
Exogenous l-Ser Modulates Action of Excitatory Amino Acid on Cerebellar Neurons.
We next examined whether the prevention of excitotoxicity (27) underlay the trophic action of l-Ser and Gly. First, we confirmed that the excessive activation of glutamate receptors was toxic to PN as reported (23, 27). NBQX and d-AP5, specific antagonists against AMPA- and NMDA-selective glutamate receptors, respectively, improved PN survival (Fig. 2B). AMPA reduced PN survival (Fig. 2C). AMPA receptors specifically mediated the AMPA toxicity because NBQX abolished the toxicity. By contrast, NMDA did not reduce PN survival, presumably because NMDA receptors could not be activated substantially in the Mg2+-containing culture medium used in this study. Second, we examined whether the activation of glutamate receptors interfered with the l-Ser trophic action. AMPA attenuated but NBQX did not enhance the l-Ser trophic action (Fig. 2D). These results suggest that the prevention of AMPA receptor-mediated toxicity partly constitutes the mechanism underlying the l-Ser trophic action. By contrast, NMDA did not attenuate the l-Ser trophic action (Fig. 2D). Both d-AP5 and MK801 (another NMDA receptor antagonist) showed additive effect to the l-Ser trophic action (Fig. 2D). These results suggest that the l-Ser trophic action is independent of NMDA receptor-mediated toxicity. Similar effects of AMPA, NMDA, NBQX, and d-AP5 were seen for the Gly trophic action (data not shown).
How do l-Ser and Gly prevent the excitotoxicity? In this regard, we found that strychnine, a Gly receptor antagonist, attenuated the l-Ser trophic action (Fig. 2E). Granule neurons in our culture system possessed strychnine-sensitive Gly receptor-mediated current (IGly) (Fig. 2F), as did those in other culture systems (28). Both l-Ser and Gly activated IGly at concentrations (≥100 μM) at which they exerted the trophic effect. IGly was carried by Cl− because its reversal potential (EGly) measured in ruptured-patch mode (−3.6 ± 2.0 mV, n = 7; data not shown) did not differ from the Cl− equilibrium potential (ECl; in this case, artificially set to 2.1 mV by the pipette solution). When measured in gramicidin-perforated-patch mode, in which the naturally occurring ECl was preserved (18), the EGly was close to the resting potential (Erest) of the granule neurons in both the early and late culture periods (Fig. 2G). Therefore, when activated by l-Ser and Gly, IGly confers an electrical force to stabilize the membrane potential of granule neurons around Erest(29). This electrical force antagonizes depolarizing events in granule neurons and consequently may reduce the depolarization-dependent synaptic release of excitatory amino acids from granule neurons (28). This reduction in the release may prevent the excitotoxicity to PN because granule neurons appeared to form abundant excitatory synapses on PN (see below).
Exogenous l-Ser Is Required for the Development of the Electrical Membrane Properties of PN.
l-Ser and Gly markedly promoted the dendritic elongation and branching of PN (red, Fig. 3 A and B). These effects appeared to be independent of synapse formation by other neurons onto PN. Immunocytostaining for synapsin I, a synaptic vesicle-associated protein demonstrated that both PN cultured with and without l-Ser for 14 days had many fluorescent puncta on their dendrites and somata (green in Fig. 3 A and B). These puncta were taken to be functional synapses because spontaneous excitatory postsynaptic currents could be recorded from all of the l-Ser-treated (n = 6) and untreated (n = 6) PN by using a patch-clamp technique (data not shown).
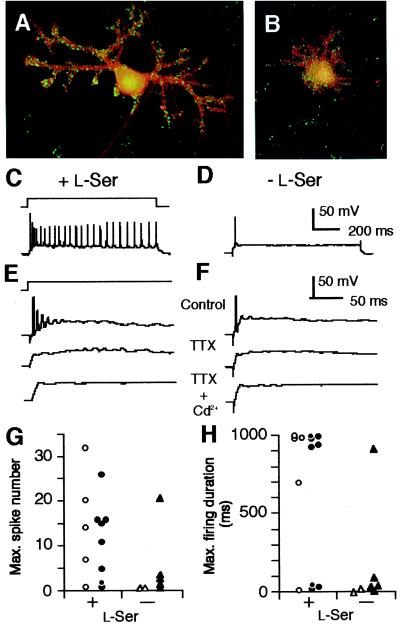
Figure 3.
Effects of exogenous l-Ser on dendritogenesis and excitability of PN. (A and B) Cultured PN maintained for 14 days in the presence (A) or absence (B) of l-Ser (100 μM) were labeled with anti-calbindin D-28K (1:500, mouse monoclonal IgG1, Sigma) and anti-synapsin I (1:200, rabbit polyclonal IgG, Chemicon) antibodies and visualized with Alexa 488 goat anti-mouse (red) and Alexa 546 anti-rabbit IgG conjugates (Molecular Probes, green), and analyzed by a confocal microscopy. (C and F) Voltage responses of PN to current pulse stimuli (schematic in C) recorded in amphotericin-perforated-patch mode. Data taken from PN cultured for 6 days in the presence (C and E) or absence (D and F) of l-Ser (100 μM). The prestimulus membrane potential was set to −65 mV by background current injection. Bath solution includes NBQX (10 μM), d-AP5 (100 μM), and labeled drugs [1 μM tetrodotoxin (TTX) and/or 0.5 mM Cd2+]. Stimulus amplitude: 100 pA in C and D; 300 pA in E and F. (G and H) Maximum number of spikes and maximum duration of firing elicited by a 1-s current pulse stimulus plotted for each cell. Data collected on DIV6–8 from PN identified morphologically (open symbols) or by immunostaining for calbindin D-28K (closed symbols). To determine the maximum number and maximum duration, stimulus amplitudes were incremented in 10-pA steps. The maximum duration was presented as the time between the pulse onset and the last spike. The percentage of neurons exhibiting sustained firing in the l-Ser-treated group (69.2%, n = 13) is significantly higher than that in the untreated group (14.3%, n = 7) (χ2 = 5.94, P = 0.0148, likelihood ratio test). The Erestand input resistance were not different between the l-Ser-treated (−48.9 ± 1.8 mV and 0.33 ± 0.07 GΩ, mean ± SEM) and untreated groups (−44.1 ± 3.6 mV and 0.33 ± 0.07 GΩ).
The effects of l-Ser and Gly in the differentiation of membrane excitability were studied by comparing voltage responses of PN cultured for 6–8 days with or without l-Ser (100 μM). When the prestimulus membrane potential was held at −65 to −70 mV (n = 20), all examined PN fired spikes in response to depolarizing current pulses (Fig. 3 C and D). The spikes consisted of fast and slow depolarizing components, which depended on tetrodotoxin-sensitive, voltage-gated Na+ current and Cd2+-sensitive, voltage-gated Ca2+ current, respectively (Fig. 3 E and F). Nine of 13 PN cultured with l-Ser fired 7 or more successive spikes during the current pulse (Fig. 3 C and G). The current intensities needed to evoke this firing varied between 20 and 900 pA and the firing persisted for at least 696 ms (Fig. 3H). In contrast, six of 7 PN in cultures without l-Ser exhibited only transient firing (Fig. 3D) regardless of the injected current intensity. This firing consisted of fewer than 5 spikes (Fig. 3G) and lasted for less than 102 ms (Fig. 3H). When cultured with Gly (200 μM), 12 of 16 PN exhibited sustained firing at DIV6–8 (data not shown). It has been reported that maturation of the firing pattern of PN, including the transition from single-spike to repetitive mode, occurs around DIV7–8 in serum- and glia-containing cultures (30, 31). Thus, our data indicate that exogenous l-Ser or Gly is important for the development of the membrane properties underlying the electrical behavior of mature PN.
3PGDH, an Enzyme Essential for l-Ser Biosynthesis, Is Expressed in the Bergmann Glia but Not in PN.
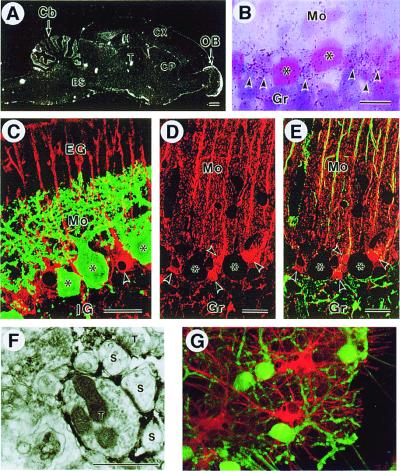
To specify the glial cell type that may serve as the main supplier of l-Ser and Gly to PN in vivo, we examined the expression of 3PGDH in the cerebellar cortex because this enzyme is indispensable for the de novo biosynthesis of the amino acids in cells (12, 13). Whole brain sections prepared with a radiolabeled probe for 3PGDH mRNA showed hybridization signals widely distributed in both the gray and white matter (Fig. 4A). The highest levels of 3PGDH mRNA occurred in the cerebellar cortex and in the olfactory nerve layer of the olfactory bulb (Fig. 4A). The labeling in the Purkinje cell layer was granular and located around larger cell bodies of PN (Fig. 4B). This localization would be consistent with a distribution of 3PGDH mRNA within the surrounding Bergmann glia. Localization of 3PGDH protein was determined by immunofluorescence and preembedding immunoelectron microscopy. In the cerebellum from 10-day-old rat, polyclonal antibodies against 3PGDH strongly labeled the entire Bergmann glia. The labeling was equally intense at the somata and the rod-like projections reaching the pial surface (Bergmann fiber) (Fig. 4C, red). Importantly, no immunoreactivity for 3PGDH was observed in calbindin-positive PN (Fig. 4C, green). A quantitative analysis using laser microscopy indicated that PN were not labeled above background levels (data not shown). In the adult rats, the polyclonal antibodies also labeled the Bergmann glia and suggested a high degree of branching from the Bergmann fibers in the molecular layer (Fig. 4D, red). No comparable labeling was detected in adult PN or granule neurons. Double immunostaining for 3PGDH and GFAP demonstrated that 3PGDH was distributed not only in GFAP-positive cell bodies and fibers of the Bergmann glia, but also in fine reticular structures that occupied the neuropil between Bergmann fibers (Fig. 4E). Immunoelectron micrographs further supported the view that the reticulated structures were Bergmann glial processes intercalated with the synapses at the dendrites of PN (Fig. 4F). The Bergmann glia may be the main source of l-Ser and Gly to PN because it is the only cell type expressing 3PGDH in the cerebellar cortex. Consistent with these results, in a glia-containing culture, the immunoreactivity for 3PGDH was detected in differentiated astroglial cells (Fig. 4G, red), but not in PN (green). PN did not show comparable expression at protein levels in vivo and in vitro at both immature and mature stages.
Figure 4.
3PGDH expression in the cerebellar cortex. (A) 3PGDH mRNA expression in the adult rat brain. Note intense expression in the cortical regions of the cerebellum (Cb) and olfactory bulb (OB). No signals are seen with a sense probe (data not shown). BS, brainstem; CP, caudate-putamen; Cx, cerebral cortex; H, hippocampus; T, thalamus. (B) Emulsion autoradiography for 3PGDH mRNA in the cerebellar cortex. Silver grains (arrowheads) were clustered around somata of PN (asterisks). Gr, granular layer; Mo, molecular layer. (C) Double immunofluorescence for 3PGDH (red) and calbindin (green) in the cerebellar cortex on the postnatal day 10. Note 3PGDH localization in the Bergmann glia (arrowhead), but not in PN (asterisks). EG, external granular layer; IG, internal granular layer. (D and E) Confocal images for 3PGDH (red) without (C) or with (D) simultaneous detection of GFAP (green) in the cerebellar cortex. Arrowheads indicate cell bodies of the Bergmann glia. Note colocalization of both immunoreactivities in Bergmann fibers running in the molecular layer, yielding an orange-to-yellow fusion color. (Scale bars: A, 1 mm; B–E, 20 μm) (F) Immunoelectron microscopy for 3PGDH in the molecular layer. Dark immunoreaction products are seen in lamellate processes of the Bergmann glia, which seal the synaptic cleft between spines of PN (S) and nerve terminals (T). (Scale bar indicates 1 μm.) (G) 3PGDH expression in cultured astroglial cells. Cerebellar mixed cultures were stained for 3PGDH (red) and calbindin D-28K (green) on DIV10. 3PGDH-positive cells were GFAP-positive astroglial cells (data not shown).
The Physiological Relevance of the Trophic Action of l-Ser and Gly.
The present finding that exogenous l-Ser and Gly are trophic for PN may be of importance also in vivo. The concentrations of l-Ser and Gly in the extracellular space of the cerebellum were estimated to be 40.5 pmol/μl and 27.8 pmol/μl, respectively (32). These levels are comparable to those at which significant trophic actions were observed in vitro (Fig. 1E). l-Ser and Gly exerted qualitatively similar trophic actions on PN (Fig. 1). This may be because these amino acids can be readily converted to each other by serine hydroxymethyltransferase, which is expressed in most mammalian cells (12, 33). The requirement of extracellular l-Ser and Gly for normal brain development in vivo has been strongly suggested by the severe delayed psychomotor development and congenital microcephaly of 3PGDH-deficient patients, in whom the two amino acids in the cerebrospinal fluid are greatly reduced (13).
l-Ser and Gly exerted their trophic action on PN cooperatively with protein trophic factors such as NT-3 and TNFα (Fig. 2A), which are synthesized by granule neurons (34) and astroglial cells (35, 36), respectively (Fig. 2A). These observations suggest that different mechanisms underlie the trophic actions of l-Ser and Gly and those of these protein factors. Our electrophysiological study indicates a possibility that l-Ser and Gly prevent the excitotoxicity to PN via the activation of Gly receptor in granule neurons (Fig. 2 F and E). However, this may explain only a minor part of the trophic actions of l-Ser and Gly (Fig. 2E). We have recently demonstrated that exogenous l-Ser or Gly is required for the de novo biosynthesis of phosphatidyl-l-serine and sphingolipids in hippocampal neurons in vitro (37). These lipids are present abundantly in PN (38–40), and the on-going biosynthesis of sphingolipids has been shown to be indispensable for the survival and dendritic differentiation of PN (39–42). Together, the role as essential metabolic substrates may largely account for the trophic actions of l-Ser and Gly on PN.
Our recent immunohistological studies have revealed the astroglia-specific expression of 3PGDH not only in the cerebellum but also in other regions of developing and mature brains of rat and human (M.Y. and M.W., unpublished work; J. Nakayama, J.M., S.F., and Y.H., unpublished work). Astroglial cells express several other enzymes involved in the metabolism of Ser and Gly. Those include serine racemase (43), serine hydroxymethyltransferase (33), and the Gly cleavage system (44). The Bergmann glia in the cerebellum expresses serine racemase abundantly (43). The present results together with these observations suggest that astroglial cells play a pivotal role in the metabolism of Ser and Gly in mammalian CNS. Our findings will bring about novel insights into the molecular communication between neuron and glia in brain.
Acknowledgments
We thank J. Tanaka for technical help, K. Takio and M. Chijimatsu for the amino acid analysis, the staff of the Animal Research Laboratory of RIKEN for the antibodies, R. T. Kado for critical reading of an early version of the manuscript, and Y. Nagai for encouragement. This work was supported by grants from the Brain Science Institute and the Frontier Research Program, RIKEN, the Ministry of Education, Science, Sports and Culture of Japan (S.F., J.M., Y.H., and M.K.), Special Coordination Funds for Promoting Science and Technology from the Science and Technology Agency (M.K. and M.W.), and a grant from the Human Frontier Science Program (M.K.).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid
- BDNF
brain-derived neurotrophic factor
- CbACM
medium conditioned by cerebral astroglia cells
- CeACM
medium conditioned by cerebellar astroglia cells
- CGCM
medium conditioned by cerebellar granule neuron-enriched cultures
- DIV
days in vitro
- GFAP
glial fibrillary acidic protein
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline
- NMDA
N-methyl-d-aspartic acid
- NT-3
neurotrophin-3
- 3PGDH
3-phosphoglycerate dehydrogenase
- PN
Purkinje neurons
- CNS
central nervous system
- TGFβ1
transforming growth factor β1
- TNFα
tumor necrosis factor α
- d-AP5
d-2-amino-5-phosphonovaleric acid
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.200364497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.200364497
References
- 1.Johnson J E. In: Fundamental Neuroscience. Zigmond M J, Bloom F E, Landis S C, Roberts J L, Squire L R, editors. San Diego: Academic; 1999. pp. 611–635. [Google Scholar]
- 2.Altman J, Bayer S A. Development of the Cerebellar System: In Relation to Its Evolution, Structure, and Functions. Boca Raton, FL: CRC Press; 1996. [Google Scholar]
- 3.Ito M. The Cerebellum and Neural Control. New York: Raven; 1984. [Google Scholar]
- 4.Hatten M E, Heintz N. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- 5.Baptista C A, Hatten M E, Blazeski R, Mason C A. Neuron. 1994;12:243–260. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 6.Morrison M E, Mason C A. J Neurosci. 1998;18:3563–3573. doi: 10.1523/JNEUROSCI.18-10-03563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brorson J R, Bleakman D, Gibbons S J, Miller R J. J Neurosci. 1991;11:4024–4043. doi: 10.1523/JNEUROSCI.11-12-04024.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuzaki M, Mikoshiba K, Kagawa Y. Biochem Biophys Res Commun. 1993;197:123–129. doi: 10.1006/bbrc.1993.2450. [DOI] [PubMed] [Google Scholar]
- 9.Mount H T, Dean D O, Alberch J, Dreyfus C F, Black I B. Proc Natl Acad Sci USA. 1995;92:9092–9096. doi: 10.1073/pnas.92.20.9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, Watanabe M. J Comp Neurol. 2000;418:106–120. [PubMed] [Google Scholar]
- 11.Colucci-Guyon E, Gimenez y Ribotta M, Maurice T, Babinet C, Privat A. Glia. 1999;25:33–43. [PubMed] [Google Scholar]
- 12.Lehninger A L. Principles of Biochemistry. New York: Worth Publishers; 1982. [Google Scholar]
- 13.Jaeken J, Detheux M, Van Maldergem L, Foulon M, Carchon H, Van Schaftingen E. Arch Dis Child. 1996;74:542–545. doi: 10.1136/adc.74.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuya S, Makino A, Hirabayashi Y. Brain Res Brain Res Protoc. 1998;3:192–198. doi: 10.1016/s1385-299x(98)00040-3. [DOI] [PubMed] [Google Scholar]
- 15.Levison S W, McCarthy K D. In: Culturing Nerve Cells. Banker G, Goslin K, editors. Cambridge, MA: MIT Press; 1994. pp. 251–281. [Google Scholar]
- 16.Mitoma J, Furuya S, Hirabayashi Y. Neurosci Res. 1998;30:195–199. doi: 10.1016/s0168-0102(97)00113-2. [DOI] [PubMed] [Google Scholar]
- 17.Marty A, Neher E. In: Single-Channel Recording. Sakmann B, Neher E, editors. New York: Plenum; 1995. pp. 31–52. [Google Scholar]
- 18.Rhee J S, Ebihara S, Akaike N. J Neurophysiol. 1994;72:1103–1108. doi: 10.1152/jn.1994.72.3.1103. [DOI] [PubMed] [Google Scholar]
- 19.Gruol D L, Crimi C P. Brain Res Dev Brain Res. 1988;41:135–146. doi: 10.1016/0165-3806(88)90177-0. [DOI] [PubMed] [Google Scholar]
- 20.Achouri Y, Rider M H, Schaftingen E V, Robbi M. Biochem J. 1997;323:365–370. doi: 10.1042/bj3230365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibata T, Yamada K, Watanabe M, Ikenaka K, Wada K, Tanaka K, Inoue Y. J Neurosci. 1997;17:9212–9219. doi: 10.1523/JNEUROSCI.17-23-09212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen-Cory S, Dreyfus C F, Black I B. J Neurosci. 1991;11:462–471. doi: 10.1523/JNEUROSCI.11-02-00462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mount H T, Dreyfus C F, Black I B. J Neurosci. 1993;13:3173–3179. doi: 10.1523/JNEUROSCI.13-07-03173.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuzaki M, Forrest D, Verselis L M, Sun S C, Curran T, Connor J A. J Neurosci. 1996;16:4651–4661. doi: 10.1523/JNEUROSCI.16-15-04651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savoca R, Ziegler U, Sonderegger P. J Neurosci Methods. 1995;61:159–167. doi: 10.1016/0165-0270(95)00038-v. [DOI] [PubMed] [Google Scholar]
- 26.Mount H T J, Dreyfus C F, Black I B. Neuroreport. 1994;5:2497–2500. doi: 10.1097/00001756-199412000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Brorson J R, Manzolillo P A, Miller R J. J Neurosci. 1994;14:187–197. doi: 10.1523/JNEUROSCI.14-01-00187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahl P, Elster L, Schousboe A. J Neurochem. 1994;62:2457–2463. doi: 10.1046/j.1471-4159.1994.62062457.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnston D, Wu S M-S. Foundations of Cellular Neurophysiology. Cambridge, MA: MIT Press; 1995. pp. 360–363. [Google Scholar]
- 30.Gruol D L, Franklin C L. J Neurosci. 1987;7:1271–1293. doi: 10.1523/JNEUROSCI.07-05-01271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller Y L, Yool A J. Dev Brain Res. 1998;108:193–203. doi: 10.1016/s0165-3806(98)00049-2. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto A, Oka T, Nishikawa T. Neuroscience. 1995;66:635–643. doi: 10.1016/0306-4522(94)00597-x. [DOI] [PubMed] [Google Scholar]
- 33.Verleysdonk S, Martin H, Willker W, Leibfritz D, Hamprecht B. Glia. 1999;27:239–248. doi: 10.1002/(sici)1098-1136(199909)27:3<239::aid-glia5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 34.Rocamora N, Garcia-Ladona F J, Palacios J M, Mengod G. Brain Res Mol Brain Res. 1993;17:1–8. doi: 10.1016/0169-328x(93)90065-w. [DOI] [PubMed] [Google Scholar]
- 35.Cheng B, Christakos S, Mattson M P. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 36.Labourdette G, Sensenbrenner M. In: Neuroglia. Kettenmann H, Ransom B R, editors. New York: Oxford Univ. Press; 1995. pp. 441–4590. [Google Scholar]
- 37.Mitoma J, Kasama T, Furuya S, Hirabayashi Y. J Biol Chem. 1998;273:19363–19366. doi: 10.1074/jbc.273.31.19363. [DOI] [PubMed] [Google Scholar]
- 38.Miyazawa A, Inoue H, Yoshioka T, Horikoshi T, Yanagisawa K, Umeda M, Inoue K. J Neurochem. 1992;59:1547–1554. doi: 10.1111/j.1471-4159.1992.tb08472.x. [DOI] [PubMed] [Google Scholar]
- 39.Furuya S, Ono K, Hirabayashi Y. J Neurochem. 1995;65:1551–1561. doi: 10.1046/j.1471-4159.1995.65041551.x. [DOI] [PubMed] [Google Scholar]
- 40.Furuya S, Mitoma J, Makino A, Hirabayashi Y. J Neurochem. 1998;71:366–377. doi: 10.1046/j.1471-4159.1998.71010366.x. [DOI] [PubMed] [Google Scholar]
- 41.Horinouchi K, Erlich S, Perl D P, Ferlinz K, Bisgaier C L, Sandhoff K, Desnick R J, Stewart C L, Schuchman E H. Nat Genet. 1995;10:288–293. doi: 10.1038/ng0795-288. [DOI] [PubMed] [Google Scholar]
- 42.Otterbach B, Stoffel W. Cell. 1995;81:1053–1061. doi: 10.1016/s0092-8674(05)80010-8. [DOI] [PubMed] [Google Scholar]
- 43.Wolosker H, Blackshaw S, Snyder S H. Proc Natl Acad Sci USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato K, Yoshida S, Fujiwara K, Tada K, Tohyama M. Brain Res. 1991;567:64–70. doi: 10.1016/0006-8993(91)91436-5. [DOI] [PubMed] [Google Scholar]