Abstract
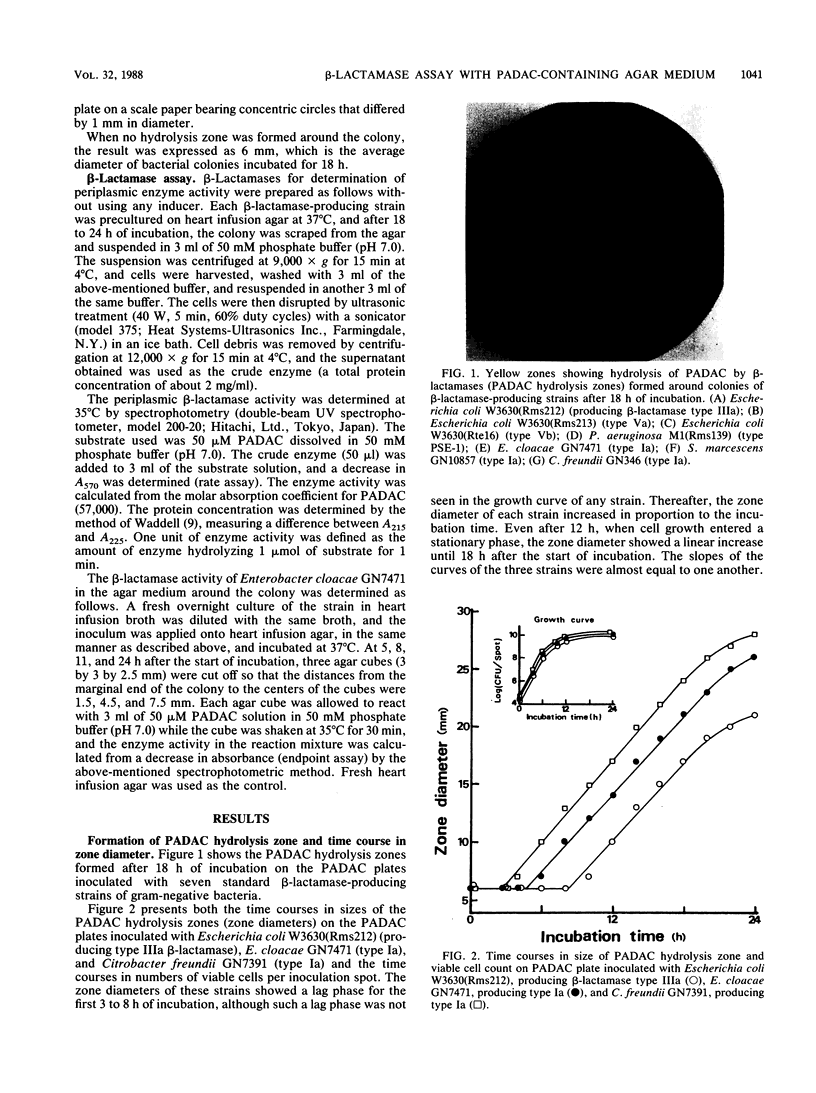
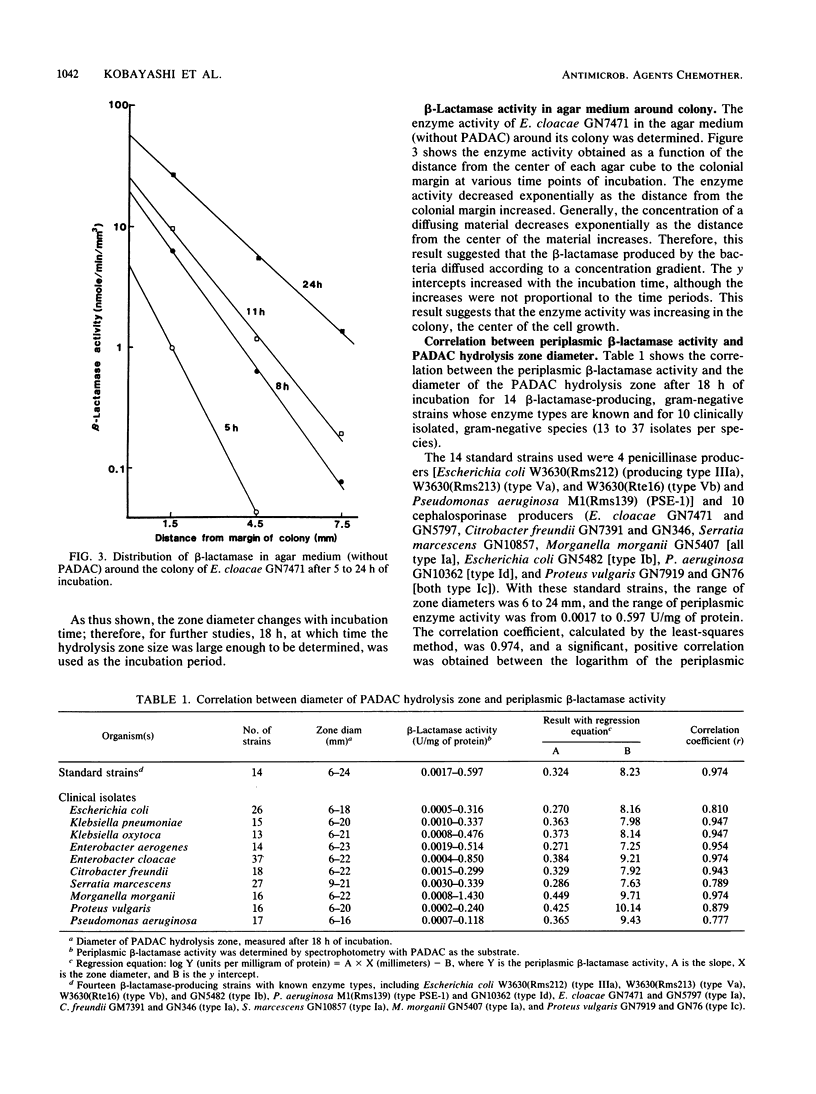
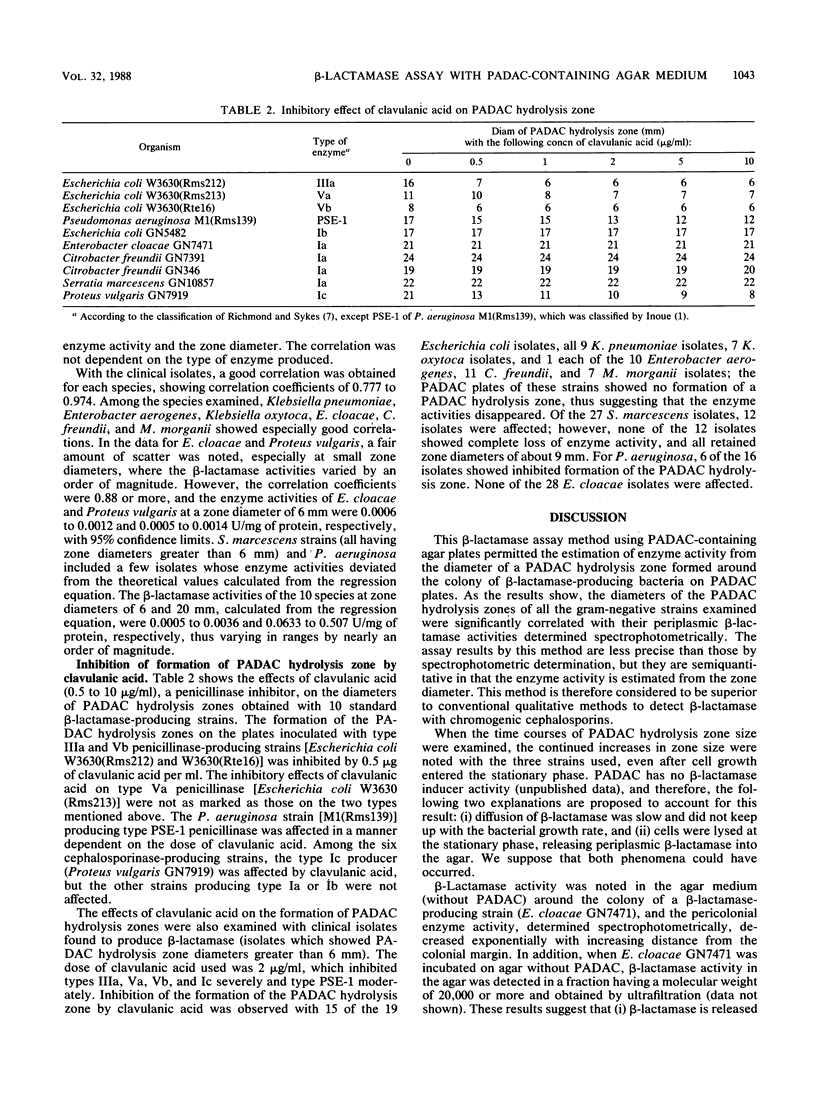
A new beta-lactamase assay method with agar plates containing pyridinium-2-azo-p-dimethylaniline chromophore (PADAC) (50 microM), a beta-lactamase-labile, chromogenic cephalosporin, was examined. On the PADAC plates inoculated with beta-lactamase-producing gram-negative bacteria (10(4) CFU per spot) and incubated at 37 degrees C, a yellow zone showing hydrolysis of PADAC by beta-lactamase was formed around the colony. The zone diameter increased with incubation time. Examination with Enterobacter cloacae GN7471 revealed that beta-lactamase activity was present in the agar around the colony, decreasing exponentially with increasing distance from the colonial margin; this suggests that the PADAC hydrolysis zone is formed by an extracellular enzyme. At 18 h, significant correlations were obtained between the zone diameters of the 10 species (clinical isolates) examined and their periplasmic beta-lactamase activities determined spectrophotometrically. The addition of clavulanic acid (0.5 to 10 micrograms/ml) inhibited zone formation on the PADAC plates inoculated with type IIIa, Va, Vb, PSE-1, and Ic beta-lactamase producers. When the clinical isolates were tested on plates with clavulanic acid (2 micrograms/ml), inhibition was observed in 41 to 58% of the Escherichia coli, Serratia marcescens, and Pseudomonas aeruginosa isolates and in all isolates of Klebsiella pneumoniae, Klebsiella oxytoca, and Proteus vulgaris. Thus, the use of the inhibitor made it possible to detect penicillinase or type Ic cephalosporinase producers. These results proved that the PADAC plate might be a useful tool permitting easy, semiquantitative determination of beta-lactamase activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jones R. N., Wilson H. W., Novick W. J., Jr In vitro evaluation of pyridine-2-azo-p-dimethylaniline cephalosporin, a new diagnostic chromogenic reagent, and comparison with nitrocefin, cephacetrile, and other beta-lactam compounds. J Clin Microbiol. 1982 Apr;15(4):677–683. doi: 10.1128/jcm.15.4.677-683.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P. Clavulanic acid, a novel inhibitor of beta-lactamases. Antimicrob Agents Chemother. 1978 Nov;14(5):650–655. doi: 10.1128/aac.14.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]




