Abstract
AIMS—To compare the clinical and molecular genetic features of two phenotypically distinct subgroups of families with type 1 Stickler syndrome. BACKGROUND—Stickler syndrome (hereditary arthro-ophthalmopathy, McKusick Nos 108300 and 184840) is a dominantly inherited disorder of collagen connective tissue, resulting in an abnormal vitreous, myopia, and a variable degree of orofacial abnormality, deafness, and arthropathy. Stickler syndrome is the commonest inherited cause of rhegmatogenous retinal detachment in childhood with a risk of giant retinal tear (GRT) which is commonly bilateral and a frequent cause of blindness. METHOD—Pedigrees were identified from the vitreoretinal service database and subclassified according to vitreoretinal phenotype. Ophthalmic, skeletal, auditory, and orofacial features were assessed. Linkage analysis was carried out with markers for the candidate genes COL2A1, COL11A1, and COL11A2. The COL2A1 gene was amplified as five overlapping PCR products. Direct sequencing of individual exons identified mutations. RESULTS—Eight families exhibiting the type 1 vitreous phenotype were studied. Seven were consistent for linkage to COL2A1, with lod scores ranging from 2.1 to 0.3. In most instances linkage to COL11A1 and COL11A2 could be excluded. One family was analysed without prior linkage analysis. Three of the families exhibited a predominantly ocular phenotype with minimal or absent systemic involvement and were found to have mutations in exon 2 of COL2A1. Five other pedigrees with an identical ocular phenotype plus orofacial, auditory, and articular involvement had mutations in others regions of the COL2A1 gene. None of the pedigrees exhibited the characteristic lenticular, retinal pigment epithelial, or choroidal changes seen in Wagner syndrome. CONCLUSIONS—These data confirm that type 1 Stickler syndrome is caused by mutations in the gene encoding type II collagen (COL2A1). In addition, data are submitted showing that mutations involving exon 2 of COL2A1 are characterised by a predominantly ocular variant of this disorder, consistent with the major form of type II procollagen in non-ocular tissues having exon 2 spliced out. Such patients are all at high risk of retinal detachment. This has important implications for counselling patients with regard to the development of systemic complications. It also emphasises the importance and reliability of the ophthalmic examination in the differential diagnosis of this predominantly ocular form of Stickler syndrome from Wagner's vitreoretinopathy.
Full Text
The Full Text of this article is available as a PDF (250.4 KB).
Figure 1 .
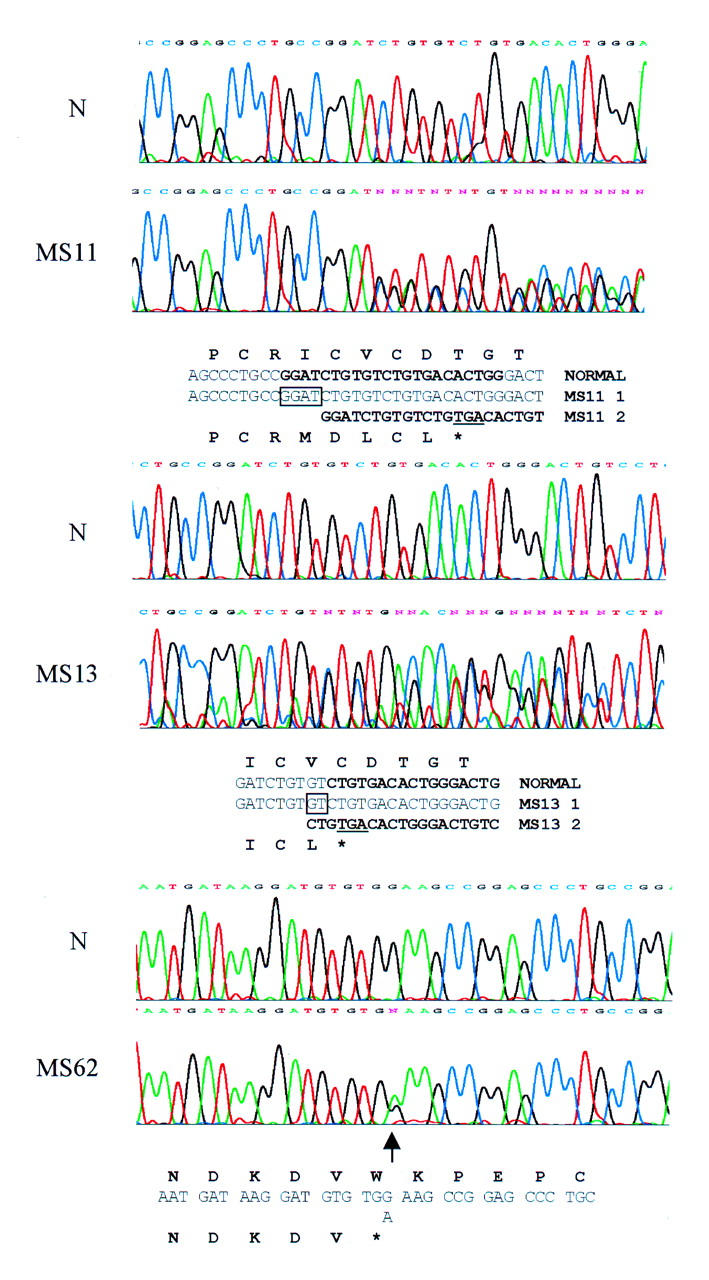
Mutation detection by sequencing. Mutations in exon 2 are shown for families MS11, MS13, and MS62 along with the normal sequence (N). The sequence for each is written under the chromatograms. For the frameshifts the mutant sequence is shown from the point at which the sequence becomes heterozygous, is in bold type, and is also indicated in the normal sequence. A 2 bp deletion and 4 bp duplication are boxed. The translated products are shown above and below the normal and mutant sequences respectively. The premature stop codons resulting from the frameshifts are underlined.
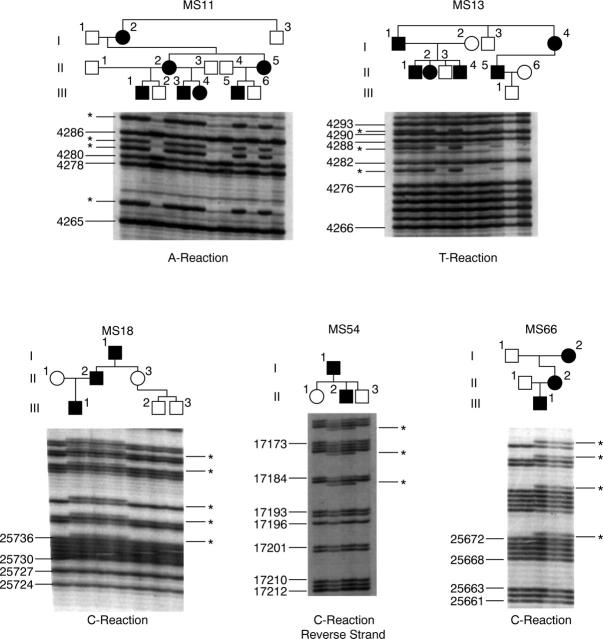
Figure 2 .
Family confirmation of frameshift mutations. 33P labelled single dideoxynucleotide, sequencing reactions (as indicated) were performed on PCR products amplified from individual family members. Numbers indicate nucleotide sequence (Accession No L10347). Asterisks indicate extra bands seen in affected family members.
Figure 3 .

Family confirmation of premature termination codons. 33P labelled single dideoxynucleotide, sequencing reactions (as indicated) were performed on PCR products amplified from individual family members. Numbers indicate nucleotide sequence (Accession No L10347). The extra bands seen in affected members are arrowed.
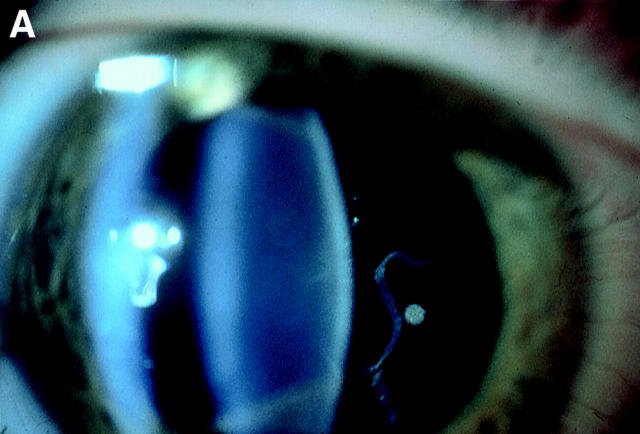
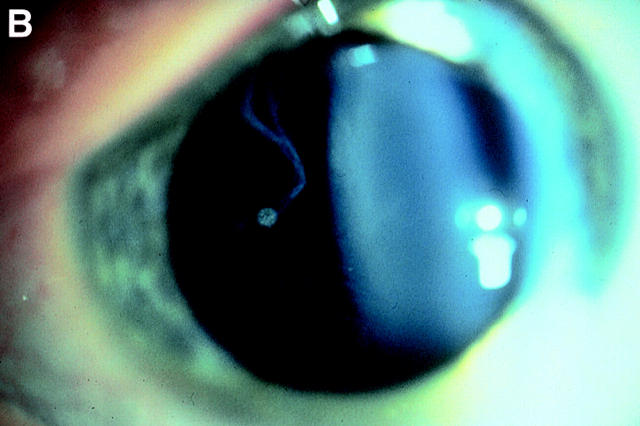
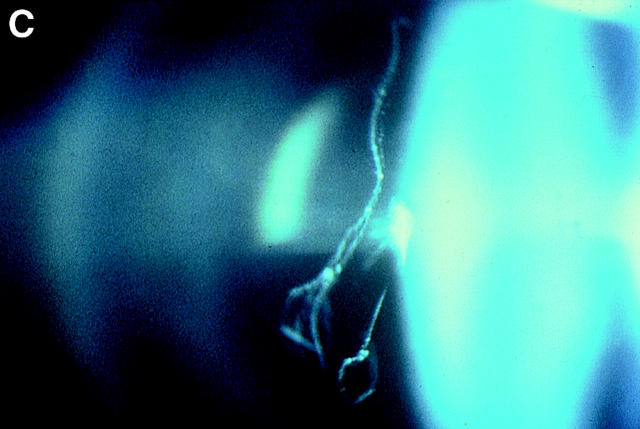
Figure 4 .
Vitreous phenotypes in Stickler syndrome. (A) Type 1 phenotype: COL2A1 non-exon 2 family MS20. (B) Type 1 phenotype: COL2A1 exon 2 family MS13. (C) Type 2 phenotype: COL11A1 mutation (data not shown, family MS42 see Martin et al7).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N. N., Ala-Kokko L., Knowlton R. G., Jimenez S. A., Weaver E. J., Maguire J. I., Tasman W., Prockop D. J. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6624–6627. doi: 10.1073/pnas.88.15.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEHRINGER H. R., DIETERLE P., LANDOLT E. [On the clinical manifestation and pathology of degeneratio hyaloideoretinalis hereditaria (Wagner)]. Ophthalmologica. 1960 Mar-Apr;139:330–338. doi: 10.1159/000303718. [DOI] [PubMed] [Google Scholar]
- Billington B. M., Leaver P. K., McLeod D. Management of retinal detachment in the Wagner-Stickler syndrome. Trans Ophthalmol Soc U K. 1985;104(Pt 8):875–879. [PubMed] [Google Scholar]
- Bishop P. N., Reardon A. J., McLeod D., Ayad S. Identification of alternatively spliced variants of type II procollagen in vitreous. Biochem Biophys Res Commun. 1994 Aug 30;203(1):289–295. doi: 10.1006/bbrc.1994.2180. [DOI] [PubMed] [Google Scholar]
- Brown D. M., Graemiger R. A., Hergersberg M., Schinzel A., Messmer E. P., Niemeyer G., Schneeberger S. A., Streb L. M., Taylor C. M., Kimura A. E. Genetic linkage of Wagner disease and erosive vitreoretinopathy to chromosome 5q13-14. Arch Ophthalmol. 1995 May;113(5):671–675. doi: 10.1001/archopht.1995.01100050139045. [DOI] [PubMed] [Google Scholar]
- Brunner H. G., van Beersum S. E., Warman M. L., Olsen B. R., Ropers H. H., Mariman E. C. A Stickler syndrome gene is linked to chromosome 6 near the COL11A2 gene. Hum Mol Genet. 1994 Sep;3(9):1561–1564. doi: 10.1093/hmg/3.9.1561. [DOI] [PubMed] [Google Scholar]
- EDMUND J. Familial retinal detachment. Acta Ophthalmol (Copenh) 1961;39:644–654. doi: 10.1111/j.1755-3768.1961.tb00279.x. [DOI] [PubMed] [Google Scholar]
- Fryer A. E., Upadhyaya M., Littler M., Bacon P., Watkins D., Tsipouras P., Harper P. S. Exclusion of COL2A1 as a candidate gene in a family with Wagner-Stickler syndrome. J Med Genet. 1990 Feb;27(2):91–93. doi: 10.1136/jmg.27.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godel V., Lazar M. Wagner's vitreoretinal degeneration with generalized epiphyseal dysplasia. Acta Ophthalmol (Copenh) 1982 Jun;60(3):469–474. doi: 10.1111/j.1755-3768.1982.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Graemiger R. A., Niemeyer G., Schneeberger S. A., Messmer E. P. Wagner vitreoretinal degeneration. Follow-up of the original pedigree. Ophthalmology. 1995 Dec;102(12):1830–1839. doi: 10.1016/s0161-6420(95)30787-7. [DOI] [PubMed] [Google Scholar]
- Horton W. A. Progress in human chondrodysplasias: molecular genetics. Ann N Y Acad Sci. 1996 Jun 8;785:150–159. doi: 10.1111/j.1749-6632.1996.tb56253.x. [DOI] [PubMed] [Google Scholar]
- Körkkö J., Ritvaniemi P., Haataja L., Käriäinen H., Kivirikko K. I., Prockop D. J., Ala-Kokko L. Mutation in type II procollagen (COL2A1) that substitutes aspartate for glycine alpha 1-67 and that causes cataracts and retinal detachment: evidence for molecular heterogeneity in the Wagner syndrome and the Stickler syndrome (arthro-ophthalmopathy) Am J Hum Genet. 1993 Jul;53(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Liberfarb R. M., Hirose T., Holmes L. B. The Wagner-Stickler syndrome: a study of 22 families. J Pediatr. 1981 Sep;99(3):394–399. doi: 10.1016/s0022-3476(81)80324-1. [DOI] [PubMed] [Google Scholar]
- Liberfarb R. M., Hirose T. The Wagner-Stickler syndrome. Birth Defects Orig Artic Ser. 1982;18(6):525–538. [PubMed] [Google Scholar]
- Martin S., Richards A. J., Yates J. R., Scott J. D., Pope M., Snead M. P. Stickler syndrome: further mutations in COL11A1 and evidence for additional locus heterogeneity. Eur J Hum Genet. 1999 Oct-Nov;7(7):807–814. doi: 10.1038/sj.ejhg.5200377. [DOI] [PubMed] [Google Scholar]
- Maumenee I. H. Vitreoretinal degeneration as a sign of generalized connective tissue diseases. Am J Ophthalmol. 1979 Sep;88(3 Pt 1):432–449. doi: 10.1016/0002-9394(79)90645-7. [DOI] [PubMed] [Google Scholar]
- Mayne R., Brewton R. G., Mayne P. M., Baker J. R. Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem. 1993 May 5;268(13):9381–9386. [PubMed] [Google Scholar]
- Monin C., Van Effenterre G., Andre-Sereys P., Haut J. Prophylaxie du décollement de rétine de la maladie de Wagner-Stickler. Etude comparative des différentes méthodes. A propos de vingt-deux cas. J Fr Ophtalmol. 1994;17(3):167–174. [PubMed] [Google Scholar]
- Perveen R., Hart-Holden N., Dixon M. J., Wiszniewski W., Fryer A. E., Brunner H. G., Pinkners A. J., van Beersum S. E., Black G. C. Refined genetic and physical localization of the Wagner disease (WGN1) locus and the genes CRTL1 and CSPG2 to a 2- to 2.5-cM region of chromosome 5q14.3. Genomics. 1999 Apr 15;57(2):219–226. doi: 10.1006/geno.1999.5766. [DOI] [PubMed] [Google Scholar]
- Pihlajamaa T., Prockop D. J., Faber J., Winterpacht A., Zabel B., Giedion A., Wiesbauer P., Spranger J., Ala-Kokko L. Heterozygous glycine substitution in the COL11A2 gene in the original patient with the Weissenbacher-Zweymüller syndrome demonstrates its identity with heterozygous OSMED (nonocular Stickler syndrome). Am J Med Genet. 1998 Nov 2;80(2):115–120. doi: 10.1002/(sici)1096-8628(19981102)80:2<115::aid-ajmg5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Richards A. J., Yates J. R., Williams R., Payne S. J., Pope F. M., Scott J. D., Snead M. P. A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in alpha 1 (XI) collagen. Hum Mol Genet. 1996 Sep;5(9):1339–1343. doi: 10.1093/hmg/5.9.1339. [DOI] [PubMed] [Google Scholar]
- STICKLER G. B., BELAU P. G., FARRELL F. J., JONES J. D., PUGH D. G., STEINBERG A. G., WARD L. E. HEREDITARY PROGRESSIVE ARTHRO-OPHTHALMOPATHY. Mayo Clin Proc. 1965 Jun;40:433–455. [PubMed] [Google Scholar]
- Sandell L. J., Morris N., Robbins J. R., Goldring M. B. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J Cell Biol. 1991 Sep;114(6):1307–1319. doi: 10.1083/jcb.114.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. D. Duke-Elder lecture. Prevention and perspective in retinal detachment. Eye (Lond) 1989;3(Pt 5):491–515. doi: 10.1038/eye.1989.82. [DOI] [PubMed] [Google Scholar]
- Sirko-Osadsa D. A., Murray M. A., Scott J. A., Lavery M. A., Warman M. L., Robin N. H. Stickler syndrome without eye involvement is caused by mutations in COL11A2, the gene encoding the alpha2(XI) chain of type XI collagen. J Pediatr. 1998 Feb;132(2):368–371. doi: 10.1016/s0022-3476(98)70466-4. [DOI] [PubMed] [Google Scholar]
- Snead M. P., Payne S. J., Barton D. E., Yates J. R., al-Imara L., Pope F. M., Scott J. D. Stickler syndrome: correlation between vitreoretinal phenotypes and linkage to COL 2A1. Eye (Lond) 1994;8(Pt 6):609–614. doi: 10.1038/eye.1994.153. [DOI] [PubMed] [Google Scholar]
- Vikkula M., Mariman E. C., Lui V. C., Zhidkova N. I., Tiller G. E., Goldring M. B., van Beersum S. E., de Waal Malefijt M. C., van den Hoogen F. H., Ropers H. H. Autosomal dominant and recessive osteochondrodysplasias associated with the COL11A2 locus. Cell. 1995 Feb 10;80(3):431–437. doi: 10.1016/0092-8674(95)90493-x. [DOI] [PubMed] [Google Scholar]
- Wilkin D. J., Mortier G. R., Johnson C. L., Jones M. C., de Paepe A., Shohat M., Wildin R. S., Falk R. E., Cohn D. H. Correlation of linkage data with phenotype in eight families with Stickler syndrome. Am J Med Genet. 1998 Nov 2;80(2):121–127. [PubMed] [Google Scholar]
- Williams C. J., Ganguly A., Considine E., McCarron S., Prockop D. J., Walsh-Vockley C., Michels V. V. A-2-->G transition at the 3' acceptor splice site of IVS17 characterizes the COL2A1 gene mutation in the original Stickler syndrome kindred. Am J Med Genet. 1996 Jun 14;63(3):461–467. doi: 10.1002/(SICI)1096-8628(19960614)63:3<461::AID-AJMG9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Zech J. C., Morlé L., Vincent P., Alloisio N., Bozon M., Gonnet C., Milazzo S., Grange J. D., Trepsat C., Godet J. Wagner vitreoretinal degeneration with genetic linkage refinement on chromosome 5q13-q14. Graefes Arch Clin Exp Ophthalmol. 1999 May;237(5):387–393. doi: 10.1007/s004170050249. [DOI] [PubMed] [Google Scholar]
- van Steensel M. A., Buma P., de Waal Malefijt M. C., van den Hoogen F. H., Brunner H. G. Oto- spondylo-megaepiphyseal dysplasia (OSMED): clinical description of three patients homozygous for a missense mutation in the COL11A2 gene. Am J Med Genet. 1997 Jun 13;70(3):315–323. doi: 10.1002/(sici)1096-8628(19970613)70:3<315::aid-ajmg19>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]