Abstract
BACKGROUND/AIMS—Familial exudative vitreoretinopathy (FEVR) is associated with mutations in the Norrie disease gene in X linked pedigrees and with linkage to the EVR1 locus at 11q13 in autosomal dominant cases. A large autosomal dominant FEVR family was studied, both clinically and by linkage analysis, to determine whether it differed from the known forms of FEVR. METHODS—Affected members and obligate gene carriers from this family were examined by slit lamp biomicroscopy, indirect ophthalmoscopy, and in some cases fluorescein angiography. Patient DNAs were genotyped for markers at the EVR1 locus on chromosome 11q13. RESULTS—The clinical evaluation in this family is consistent with previous descriptions of FEVR pedigrees, but linkage analysis proves that it has a form of FEVR genetically distinct from the EVR1 locus on 11q. CONCLUSION—This proves that there are at least three different loci associated with comparable FEVR phenotypes, a situation similar to that existing for many forms of retinal degeneration.
Full Text
The Full Text of this article is available as a PDF (159.3 KB).
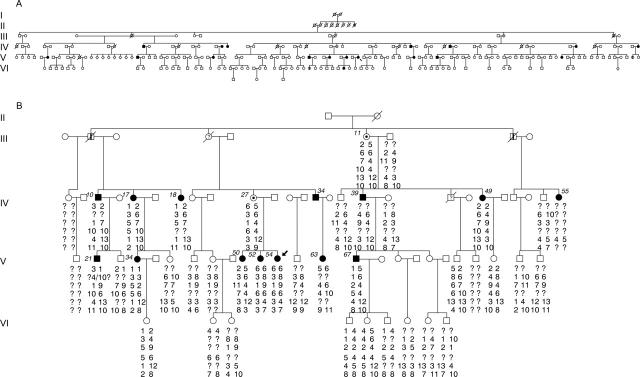
Figure 1 .
The pedigree of the familial exudative vitreoretinopathy (FEVR) family described, segregating autosomal dominant FEVR, drawn using the program CYRILLIC 2. (A) A complete family tree, but many of the apparently normal individuals have not been examined by an ophthalmologist and the subtle nature of the FEVR phenotype in some cases is such that carrier status cannot be excluded. Solid symbols are confirmed as affected by clinical examination, those marked with a vertical line are said to be or to have been affected, those marked with a dot are asymptomatic obligate carriers, and those marked with a question mark have an uncertain diagnosis which is being investigated further. (B) A subset of (A) which has been subject to genetic analysis, and haplotypes for 11q markers are given below each symbol (reading downwards the haplotypes are obtained from markers D11S916, D11S527, D11S937, D11S1396, D11SS873, and D11S876). Affected individuals are also numbered (italic) to correspond with (A) and with Table 1.
Figure 2 .
Fundus photographs of the left and right eyes of the proband. (A) Right eye: temporal periphery shows white preretinal mass with retinal traction. This eye has previously undergone a pars plana vitrectomy together with endophotocoagulation (the scars from which can also be seen here). (B) Left eye: distortion of major retinal vessels at the optic disc due to vitreoretinal traction.
Figure 3 .
Multipoint lod scores from two separate analyses carried out on data from this pedigree, using the program LINKMAP, from the LINKAGE suit of programs. Penetrance was assumed to be 80% to allow for the possibility that some apparently normal individuals might in fact be carriers. The first analysis (points for which are marked as solid squares) used data from markers D11S916, D11S937, and D11S1396 against disease status. The second, which overlaps the first and is marked as a series of open circles, uses data from D11S1396, D11S873, and D11S876. Approximate positions of the EVR1 FEVR locus and the VRN1 ADNIV locus are shown above. Distances are a composite of those derived from several mapping sources.12 13 24 27-29 Genetic distances along the Y axis are given in centimorgans (cM) from marker D11S916.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascom R. A., Liu L., Heckenlively J. R., Stone E. M., McInnes R. R. Mutation analysis of the ROM1 gene in retinitis pigmentosa. Hum Mol Genet. 1995 Oct;4(10):1895–1902. doi: 10.1093/hmg/4.10.1895. [DOI] [PubMed] [Google Scholar]
- Bennett S. R., Folk J. C., Kimura A. E., Russell S. R., Stone E. M., Raphtis E. M. Autosomal dominant neovascular inflammatory vitreoretinopathy. Ophthalmology. 1990 Sep;97(9):1125–1136. doi: 10.1016/s0161-6420(90)32447-8. [DOI] [PubMed] [Google Scholar]
- Benson W. E. Familial exudative vitreoretinopathy. Trans Am Ophthalmol Soc. 1995;93:473–521. [PMC free article] [PubMed] [Google Scholar]
- Boldrey E. E., Egbert P., Gass J. D., Friberg T. The histopathology of familial exudative vitreoretinopathy. A report of two cases. Arch Ophthalmol. 1985 Feb;103(2):238–241. doi: 10.1001/archopht.1985.01050020090029. [DOI] [PubMed] [Google Scholar]
- Chen Z. Y., Battinelli E. M., Fielder A., Bundey S., Sims K., Breakefield X. O., Craig I. W. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet. 1993 Oct;5(2):180–183. doi: 10.1038/ng1093-180. [DOI] [PubMed] [Google Scholar]
- Criswick V. G., Schepens C. L. Familial exudative vitreoretinopathy. Am J Ophthalmol. 1969 Oct;68(4):578–594. doi: 10.1016/0002-9394(69)91237-9. [DOI] [PubMed] [Google Scholar]
- Ebert E. M., Mukai S. Familial exudative vitreoretinopathy. Int Ophthalmol Clin. 1993 Spring;33(2):237–247. doi: 10.1097/00004397-199303320-00022. [DOI] [PubMed] [Google Scholar]
- Espinós C., Nájera C., Millán J. M., Ayuso C., Baiget M., Pérez-Garrigues H., Rodrigo O., Vilela C., Beneyto M. Linkage analysis in Usher syndrome type I (USH1) families from Spain. J Med Genet. 1998 May;35(5):391–398. doi: 10.1136/jmg.35.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullwood P., Jones J., Bundey S., Dudgeon J., Fielder A. R., Kilpatrick M. W. X linked exudative vitreoretinopathy: clinical features and genetic linkage analysis. Br J Ophthalmol. 1993 Mar;77(3):168–170. doi: 10.1136/bjo.77.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni-Baruch R., Rosenmann A., Droetto S., Holmes S., Tripathi R. K., Spritz R. A. Mutations of the tyrosinase gene in patients with oculocutaneous albinism from various ethnic groups in Israel. Am J Hum Genet. 1994 Apr;54(4):586–594. [PMC free article] [PubMed] [Google Scholar]
- Gow J., Oliver G. L. Familial exudative vitreoretinopathy. An expanded view. Arch Ophthalmol. 1971 Aug;86(2):150–155. doi: 10.1001/archopht.1971.01000010152007. [DOI] [PubMed] [Google Scholar]
- Inglehearn C. F. Intelligent linkage analysis using gene density estimates. Nat Genet. 1997 May;16(1):15–15. doi: 10.1038/ng0597-15. [DOI] [PubMed] [Google Scholar]
- Inglehearn C. F. Molecular genetics of human retinal dystrophies. Eye (Lond) 1998;12(Pt 3B):571–579. doi: 10.1038/eye.1998.147. [DOI] [PubMed] [Google Scholar]
- Kajiwara K., Berson E. L., Dryja T. P. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994 Jun 10;264(5165):1604–1608. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- Leppert M., Baird L., Anderson K. L., Otterud B., Lupski J. R., Lewis R. A. Bardet-Biedl syndrome is linked to DNA markers on chromosome 11q and is genetically heterogeneous. Nat Genet. 1994 May;7(1):108–112. doi: 10.1038/ng0594-108. [DOI] [PubMed] [Google Scholar]
- Li Y., Müller B., Fuhrmann C., van Nouhuys C. E., Laqua H., Humphries P., Schwinger E., Gal A. The autosomal dominant familial exudative vitreoretinopathy locus maps on 11q and is closely linked to D11S533. Am J Hum Genet. 1992 Oct;51(4):749–754. [PMC free article] [PubMed] [Google Scholar]
- Miyakubo H., Hashimoto K., Miyakubo S. Retinal vascular pattern in familial exudative vitreoretinopathy. Ophthalmology. 1984 Dec;91(12):1524–1530. doi: 10.1016/s0161-6420(84)34119-7. [DOI] [PubMed] [Google Scholar]
- Miyakubo H., Inohara N., Hashimoto K. Retinal involvement in familial exudative vitreoretinopathy. Ophthalmologica. 1982;185(3):125–135. doi: 10.1159/000309234. [DOI] [PubMed] [Google Scholar]
- Müller B., Orth U., van Nouhuys C. E., Duvigneau C., Fuhrmann C., Schwinger E., Laqua H., Gal A. Mapping of the autosomal dominant exudative vitreoretinopathy locus (EVR1) by multipoint linkage analysis in four families. Genomics. 1994 Mar 15;20(2):317–319. doi: 10.1006/geno.1994.1176. [DOI] [PubMed] [Google Scholar]
- Ober R. R., Bird A. C., Hamilton A. M., Sehmi K. Autosomal dominant exudative vitreoretinopathy. Br J Ophthalmol. 1980 Feb;64(2):112–120. doi: 10.1136/bjo.64.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrukhin K., Koisti M. J., Bakall B., Li W., Xie G., Marknell T., Sandgren O., Forsman K., Holmgren G., Andreasson S. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998 Jul;19(3):241–247. doi: 10.1038/915. [DOI] [PubMed] [Google Scholar]
- Shastry B. S., Hejtmancik J. F., Plager D. A., Hartzer M. K., Trese M. T. Linkage and candidate gene analysis of X-linked familial exudative vitreoretinopathy. Genomics. 1995 May 20;27(2):341–344. doi: 10.1006/geno.1995.1052. [DOI] [PubMed] [Google Scholar]
- Shastry B. S., Hejtmancik J. F., Trese M. T. Identification of novel missense mutations in the Norrie disease gene associated with one X-linked and four sporadic cases of familial exudative vitreoretinopathy. Hum Mutat. 1997;9(5):396–401. doi: 10.1002/(SICI)1098-1004(1997)9:5<396::AID-HUMU3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Shastry B. S., Trese M. T. Familial exudative vitreoretinopathy: further evidence for genetic heterogeneity. Am J Med Genet. 1997 Mar 17;69(2):217–218. doi: 10.1002/(sici)1096-8628(19970317)69:2<217::aid-ajmg19>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Lee E. C., Kimberling W. J., Daiger S. P., Pelias M. Z., Keats B. J., Jay M., Bird A., Reardon W., Guest M. Localization of two genes for Usher syndrome type I to chromosome 11. Genomics. 1992 Dec;14(4):995–1002. doi: 10.1016/s0888-7543(05)80122-3. [DOI] [PubMed] [Google Scholar]
- Stone E. M., Kimura A. E., Folk J. C., Bennett S. R., Nichols B. E., Streb L. M., Sheffield V. C. Genetic linkage of autosomal dominant neovascular inflammatory vitreoretinopathy to chromosome 11q13. Hum Mol Genet. 1992 Dec;1(9):685–689. doi: 10.1093/hmg/1.9.685. [DOI] [PubMed] [Google Scholar]
- de Crecchio G., Simonelli F., Nunziata G., Mazzeo S., Greco G. M., Rinaldi E., Ventruto V., Ciccodicola A., Miano M. G., Testa F. Autosomal recessive familial exudative vitreoretinopathy: evidence for genetic heterogeneity. Clin Genet. 1998 Oct;54(4):315–320. doi: 10.1034/j.1399-0004.1998.5440409.x. [DOI] [PubMed] [Google Scholar]
- van Nouhuys C. E. Dominant exudative vitreoretinopathy and other vascular developmental disorders of the peripheral retina. Doc Ophthalmol. 1982 Sep 23;54(1-4):1–414. doi: 10.1007/BF00183127. [DOI] [PubMed] [Google Scholar]