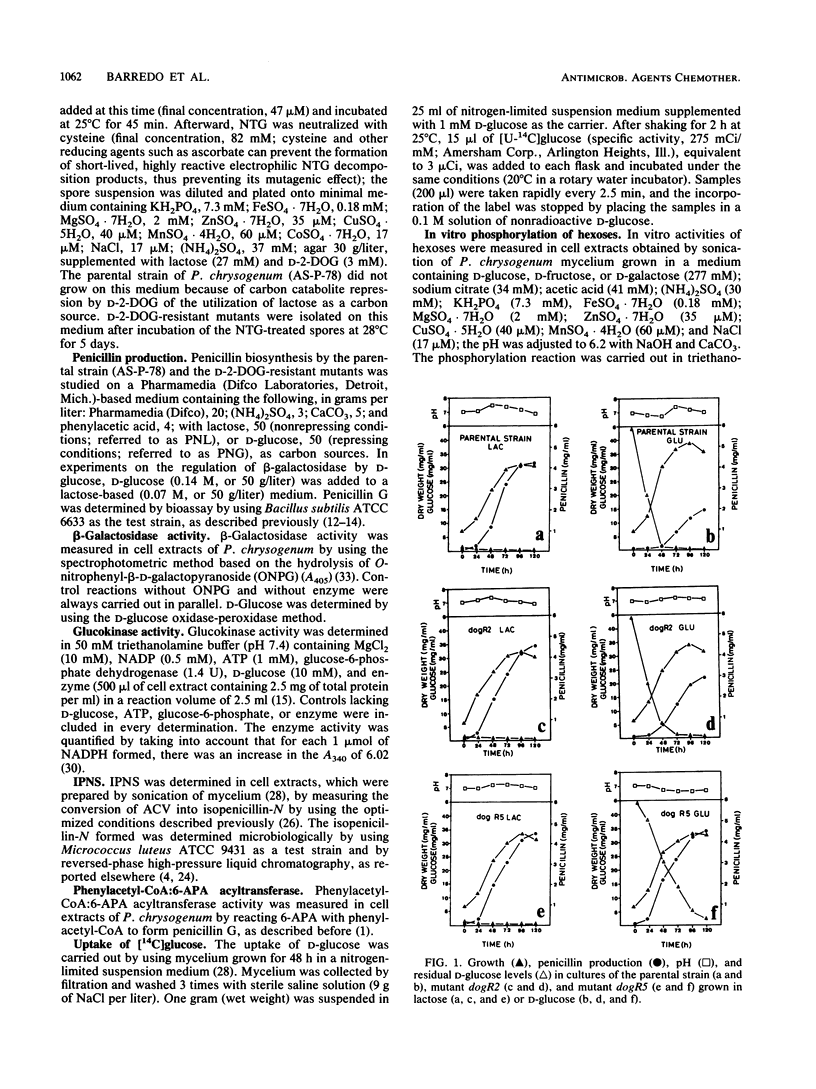
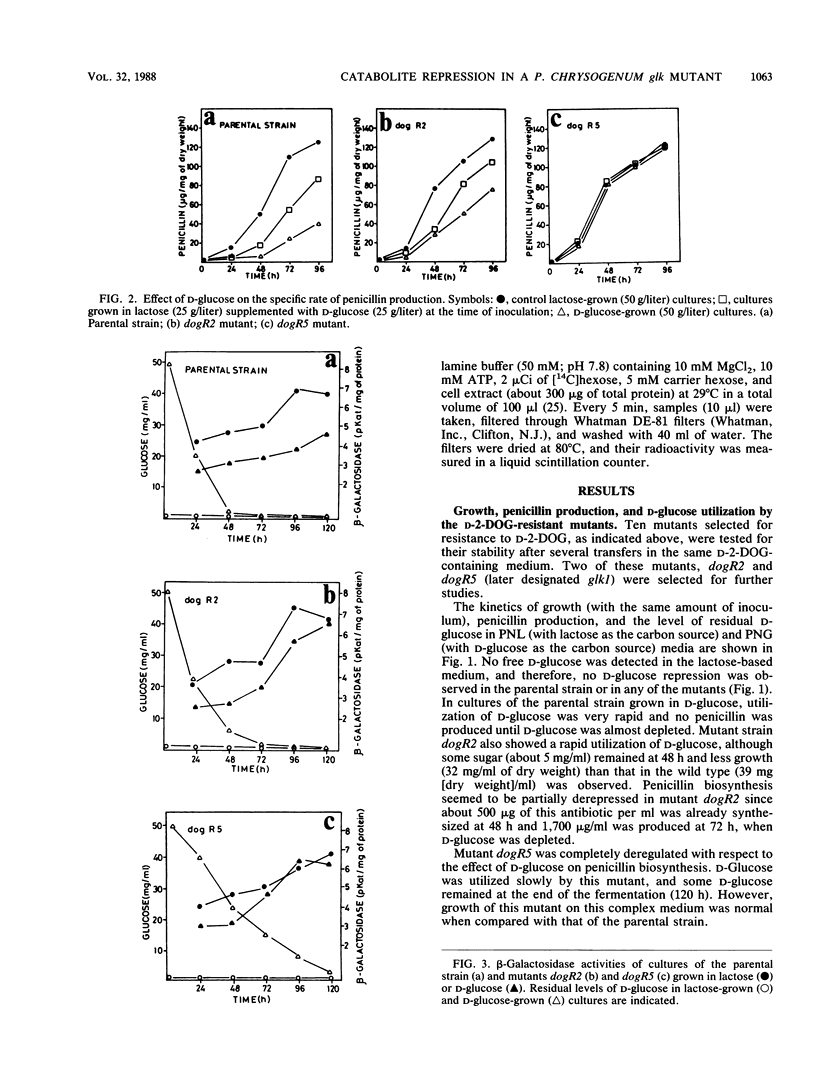
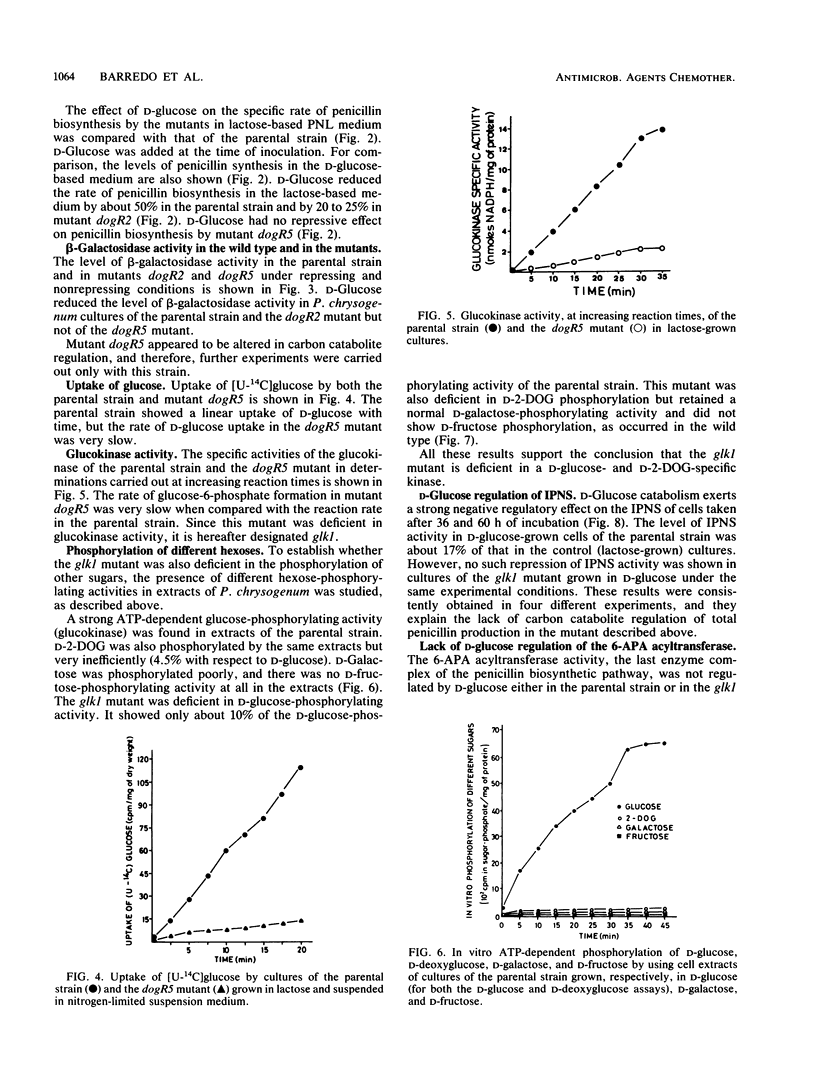
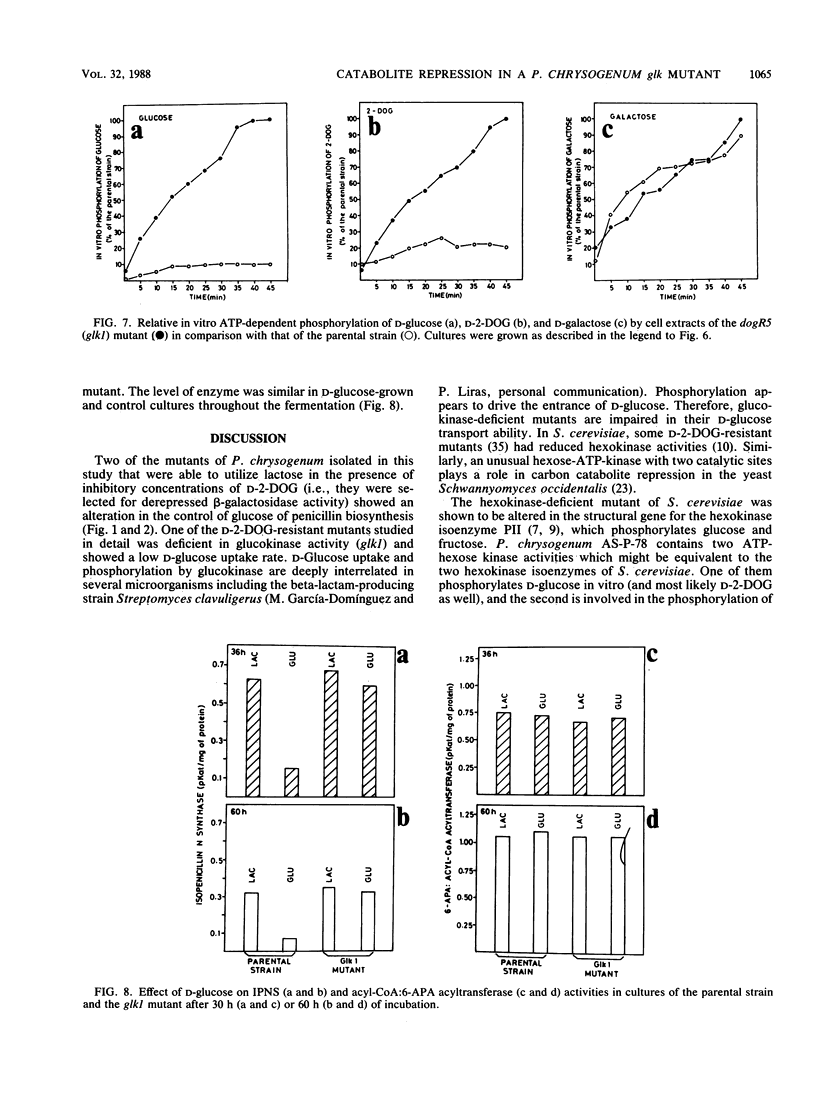
Abstract
One glucokinase-deficient mutant (glk1) of Penicillium chrysogenum AS-P-78 was isolated after germ tube-emitting spores were mutated with nitrosoguanidine and selected for growth on lactose-containing medium in the presence of inhibitory concentrations of D-2-deoxyglucose (3 mM). Penicillin biosynthesis was greatly reduced (55%) in D-glucose-grown cultures of the parental strain, but this sugar had no repressive effect on the rate of penicillin biosynthesis in the mutant glk1. This mutant was deficient in ATP-dependent glucokinase and showed a greatly reduced uptake of D-glucose. The parental strain P. chrysogenum AS-P-78 showed in vitro ATP-dependent phosphorylating activities of D-glucose, D-2-deoxyglucose, and D-galactose. The glk1 mutant was deficient in the in vitro phosphorylation of D-glucose and D-2-deoxyglucose but retained a normal D-galactose-phosphorylating activity. D-Glucose repressed both beta-galactosidase and isopenicillin-N-synthase but not acyl coenzyme A:6-aminopenicillanic acid acyltransferase in the parental strain. The glucokinase-deficient mutant was simultaneously derepressed in carbon catabolite regulation of beta-galactosidase and isopenicillin-N-synthase, suggesting that a common regulatory mechanism is involved in carbon catabolite regulation of both sugar utilization and penicillin biosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez E., Cantoral J. M., Barredo J. L., Díez B., Martín J. F. Purification to homogeneity and characterization of acyl coenzyme A:6-aminopenicillanic acid acyltransferase of Penicillium chrysogenum. Antimicrob Agents Chemother. 1987 Nov;31(11):1675–1682. doi: 10.1128/aac.31.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beri R. K., Turner G. Transformation of Penicillium chrysogenum using the Aspergillus nidulans amdS gene as a dominant selective marker. Curr Genet. 1987;11(8):639–641. doi: 10.1007/BF00393928. [DOI] [PubMed] [Google Scholar]
- Castro J. M., Liras P., Laíz L., Cortés J., Martín J. F. Purification and characterization of the isopenicillin N synthase of Streptomyces lactamdurans. J Gen Microbiol. 1988 Jan;134(1):133–141. doi: 10.1099/00221287-134-1-133. [DOI] [PubMed] [Google Scholar]
- Cortés J., Liras P., Castro J. M., Martín J. F. Glucose regulation of cephamycin biosynthesis in Streptomyces lactamdurans is exerted on the formation of alpha-aminoadipyl-cysteinyl-valine and deacetoxycephalosporin C synthase. J Gen Microbiol. 1986 Jul;132(7):1805–1814. doi: 10.1099/00221287-132-7-1805. [DOI] [PubMed] [Google Scholar]
- Cortés J., Martín J. F., Castro J. M., Láiz L., Liras P. Purification and characterization of a 2-oxoglutarate-linked ATP-independent deacetoxycephalosporin C synthase of Streptomyces lactamdurans. J Gen Microbiol. 1987 Nov;133(11):3165–3174. doi: 10.1099/00221287-133-11-3165. [DOI] [PubMed] [Google Scholar]
- Entian K. D. Genetic and biochemical evidence for hexokinase PII as a key enzyme involved in carbon catabolite repression in yeast. Mol Gen Genet. 1980;178(3):633–637. doi: 10.1007/BF00337871. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Kopetzki E., Fröhlich K. U., Mecke D. Cloning of hexokinase isoenzyme PI from Saccharomyces cerevisiae: PI transformants confirm the unique role of hexokinase isoenzyme PII for glucose repression in yeasts. Mol Gen Genet. 1984;198(2):50–54. doi: 10.1007/BF00328699. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Mecke D. Genetic evidence for a role of hexokinase isozyme PII in carbon catabolite repression in Saccharomyces cerevisiae. J Biol Chem. 1982 Jan 25;257(2):870–874. [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K., Scheel I. A partial defect in carbon catabolite repression in mutants of Saccharomyces cerevisiae with reduced hexose phosphyorylation. Mol Gen Genet. 1977 Nov 4;156(1):99–105. doi: 10.1007/BF00272258. [DOI] [PubMed] [Google Scholar]
- Gancedo C., Schwerzmann K. Inactivation by glucose of phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae. Arch Microbiol. 1976 Sep 1;109(3):221–225. doi: 10.1007/BF00446632. [DOI] [PubMed] [Google Scholar]
- Luengo J. M., Revilla G., Villanueva J. R., Martín J. F. Lysine regulation of penicillin biosynthesis in low-producing and industrial strains of Penicillium chrysogenum. J Gen Microbiol. 1979 Nov;115(1):207–211. doi: 10.1099/00221287-115-1-207. [DOI] [PubMed] [Google Scholar]
- Maitra P. K. A glucokinase from Saccharomyces cerevisiae. J Biol Chem. 1970 May 10;245(9):2423–2431. [PubMed] [Google Scholar]
- Martin-Zanca D. M., Martín J. F. Carbon catabolite regulation of the conversion of penicillin N into cephalosporin C. J Antibiot (Tokyo) 1983 Jun;36(6):700–708. doi: 10.7164/antibiotics.36.700. [DOI] [PubMed] [Google Scholar]
- Martin J. F., Demain A. L. Control of antibiotic biosynthesis. Microbiol Rev. 1980 Jun;44(2):230–251. doi: 10.1128/mr.44.2.230-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. F., Liras P., Villanueva J. R. Changes in composition of conidia of Penicillium notatum during germination. Arch Mikrobiol. 1974 Apr 10;97(1):39–50. doi: 10.1007/BF00403043. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T., Oshima Y. Cyclic AMP may not be involved in catabolite repression in Saccharomyces cerevisiae: evidence from mutants unable to synthesize it. J Bacteriol. 1983 Nov;156(2):898–900. doi: 10.1128/jb.156.2.898-900.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F. R., López-Nieto M. J., Martín J. F. Isopenicillin N synthetase of Penicillium chrysogenum, an enzyme that converts delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine to isopenicillin N. Antimicrob Agents Chemother. 1985 Mar;27(3):380–387. doi: 10.1128/aac.27.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla G., López-Nieto M. J., Luengo J. M., Martín J. F. Carbon catabolite repression of penicillin biosynthesis by Penicillium chrysogenum. J Antibiot (Tokyo) 1984 Jul;37(7):781–789. doi: 10.7164/antibiotics.37.781. [DOI] [PubMed] [Google Scholar]
- Revilla G., Ramos F. R., López-Nieto M. J., Alvarez E., Martín J. F. Glucose represses formation of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine and isopenicillin N synthase but not penicillin acyltransferase in Penicillium chrysogenum. J Bacteriol. 1986 Nov;168(2):947–952. doi: 10.1128/jb.168.2.947-952.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno E. T., Chater K. F. Glycerol catabolic enzymes and their regulation in wild-type and mutant strains of Streptomyces coelicolor A3(2). J Gen Microbiol. 1983 May;129(5):1403–1413. doi: 10.1099/00221287-129-5-1403. [DOI] [PubMed] [Google Scholar]
- Sánchez F., Lozano M., Rubio V., Peñalva M. A. Transformation in Penicillium chrysogenum. Gene. 1987;51(1):97–102. doi: 10.1016/0378-1119(87)90479-3. [DOI] [PubMed] [Google Scholar]
- Van Wijk R., Ouwehand J., van den Bos T., Koningsberger V. V. Induction and catabolite repression of alpha-glucosidase synthesis in protoplasts of Saccharomyces carlsbergensis. Biochim Biophys Acta. 1969 Jul 22;186(1):178–191. doi: 10.1016/0005-2787(69)90501-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Scheel I. Mutants of Saccharomyces cerevisiae resistant to carbon catabolite repression. Mol Gen Genet. 1977 Jul 7;154(1):75–82. doi: 10.1007/BF00265579. [DOI] [PubMed] [Google Scholar]


