Abstract
AIM—To determine the relative impact of CD4+ T cells and CD8+ T cells in protecting mice against ocular HSV-1 challenge. METHODS—CD4+ T cell knockout mice (CD4−/− mice), CD8+ T cell knockout mice (CD8−/− mice), and mice depleted for CD4+ or CD8+ T cells by antibody (CD4+ depleted and CD8+ depleted mice), were examined for their ability to withstand HSV-1 ocular challenge. The parental mice for both knockout mice were C57BL/6J. RESULTS—These results suggest that: (1) both CD4+ deficient mice (CD4−/− and CD4+ depleted mice) and CD8+ deficient mice (CD8−/−, and CD8+ depleted mice) developed significantly more corneal scarring than their C57BL/6J parental strain; (2) the duration of virus clearance from the eyes of the CD4+ deficient mice was 4 days longer than that of the CD8+ deficient mice; and (3) the severity of corneal scarring in the CD4+ deficient mice was approximately twice that of the CD8+ deficient mice. CONCLUSIONS—It was reported here that: (1) CD4+ and CD8+ T cells were both involved in protection against lethal ocular HSV-1 infection; and (2) CD4+ and CD8+ T cells were both involved in protection against HSV-1 induced corneal scarring.
Full Text
The Full Text of this article is available as a PDF (126.7 KB).
Figure 1 .
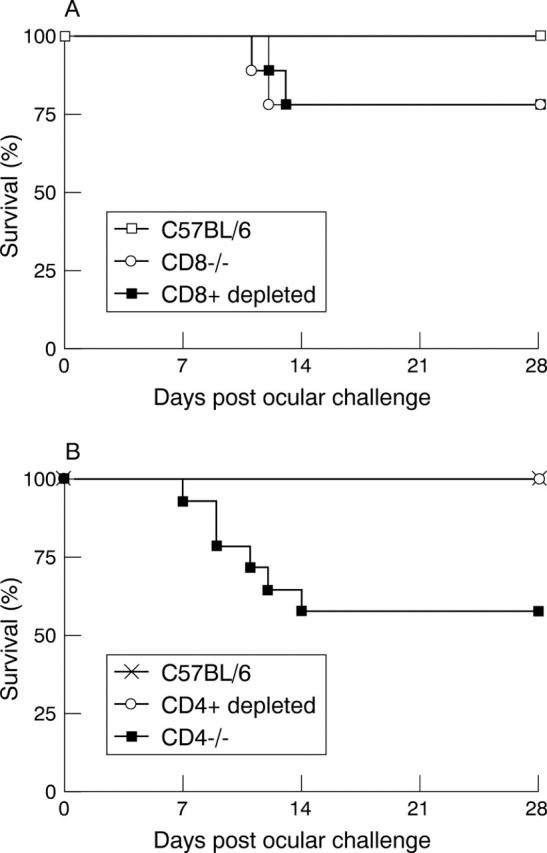
Survival of T cell deficient mice. Mice were inoculated ocularly with 2×106 pfu of McKrae as described in Materials and method. Survival was measured 28 days after ocular challenge. (A) Survival of CD8−/− and CD8+ depleted mice compared with C57BL/6J control mice after ocular infection with HSV-1. CD8−/− mice (n=10), CD8+ depleted (n=9), and C57BL/6J (n=9). (B) Survival of CD4−/− and CD4+ depleted mice compared with C57BL/6J control mice after ocular infection with HSV-1. CD4−/− mice (n=14), CD4+ depleted (n=9), and C57BL/6J (n=10).
Figure 2 .
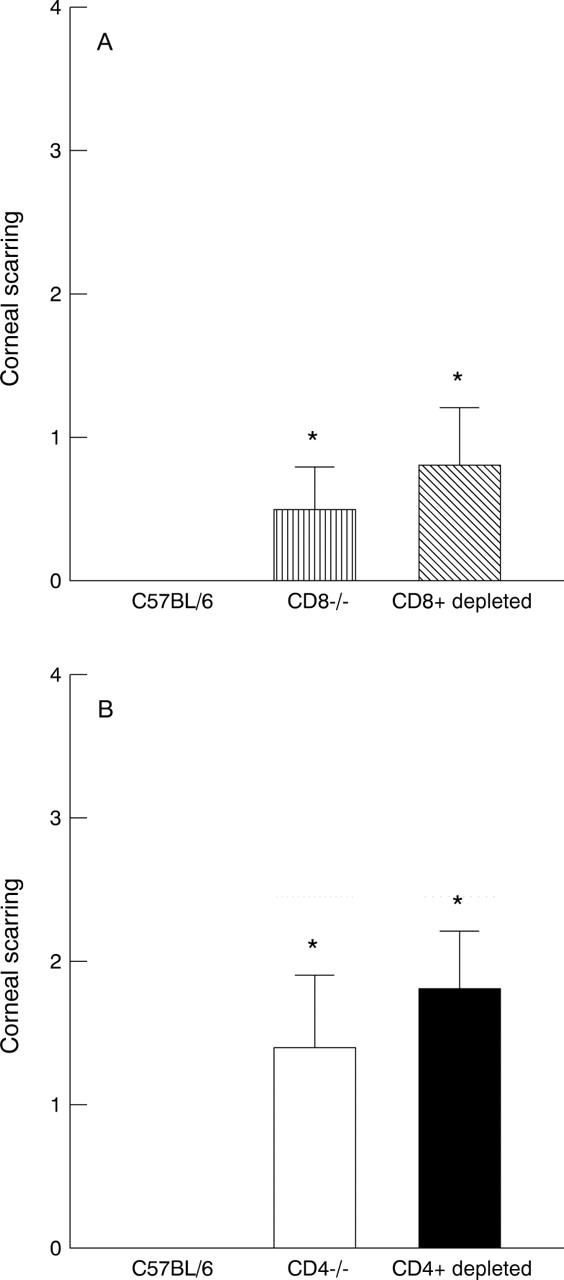
Corneal scarring in T cell deficient mice. Corneal scarring was measured 28 days after ocular challenge as described in Materials and method in the mice shown in Figure 1 that survived ocular challenge. (A) Corneal scarring in CD8+ deficient mice; (B) corneal scarring in CD4+ deficient mice. For each bar, the corneal scarring (y axis) represents the average from 16 eyes (CD8−/− mice), 14 eyes (CD8+ depleted mice), 20 eyes (C57BL/6 mice), 16 eyes (CD4−/− mice), 18 eyes (CD4+ depleted mice), and 20 eyes (C57BL/6 mice) (SEM). *Similar to each other and significantly different from other groups (p<0.001, Student's t test).
Figure 3 .
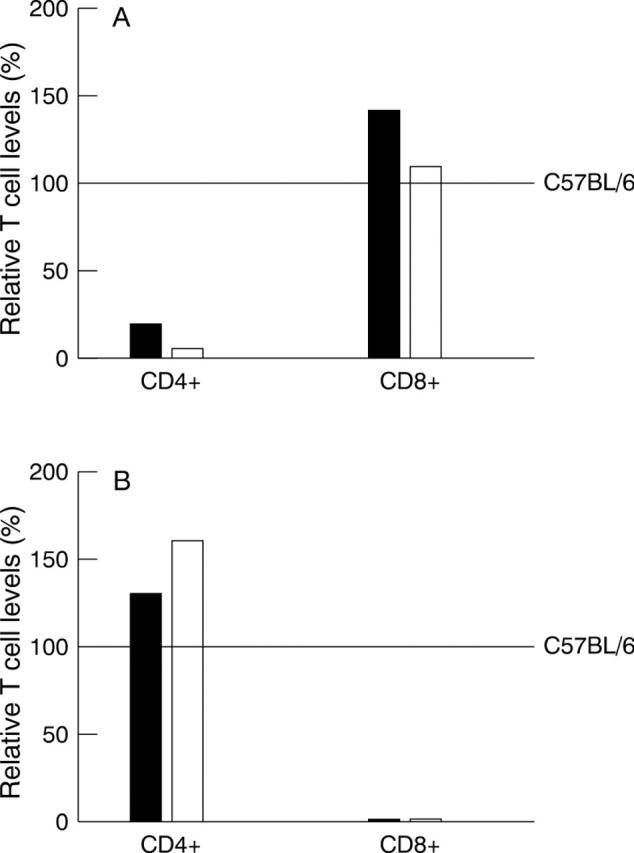
Cytofluorimetric analysis of spleen cells from T cell deficient mice. (A) Spleens from two CD4−/− mice (solid bars) or two C57BL/6 mice depleted for CD4+ T cells with antibody (open bars) were harvested. Single cell suspensions of splenocytes were prepared, reacted with mAbs to CD4+ T cells (L3T4 mAb) and CD8+ T cells (Lyt-2 mAb) and FACS analysis was performed as described in Materials and methods. The level of CD4+CD8− T cells (CD4+) and the level of CD4−CD8+ T cells (CD8+) are shown relative to the level of these cells in untreated control C57BL/6 mice (horizontal line at 100%). Each bar represents the mean from two to four FACS analyses. (B) Spleen cells from two CD8−/− mice (solid bars) or two C57BL/6 mice depleted for CD8+ T cells with antibody (open bars) were prepared and FACS analysis performed as in (A).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battegay M., Moskophidis D., Rahemtulla A., Hengartner H., Mak T. W., Zinkernagel R. M. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994 Jul;68(7):4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. R. Susceptibility of +/+, +/nu and nu/nu BALB/c mice to ocular herpes simplex virus infection. Ophthalmic Res. 1992;24(6):332–337. doi: 10.1159/000267189. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Hou S., Southern P. J. Lymphocytic choriomeningitis virus induces a chronic wasting disease in mice lacking class I major histocompatibility complex glycoproteins. J Neuroimmunol. 1993 Jul;46(1-2):11–17. doi: 10.1016/0165-5728(93)90228-q. [DOI] [PubMed] [Google Scholar]
- Doymaz M. Z., Rouse B. T. Herpetic stromal keratitis: an immunopathologic disease mediated by CD4+ T lymphocytes. Invest Ophthalmol Vis Sci. 1992 Jun;33(7):2165–2173. [PubMed] [Google Scholar]
- Easty D. L., Shimeld C., Claoue C. M., Menage M. Herpes simplex virus isolation in chronic stromal keratitis: human and laboratory studies. Curr Eye Res. 1987 Jan;6(1):69–74. doi: 10.3109/02713688709020071. [DOI] [PubMed] [Google Scholar]
- Foster C. S., Sandstrom I. K., Wells P. A., Thompson P., Daigle J., Opremcak E. M. Immunomodulation of experimental murine herpes simplex keratitis: II. Glycoprotein D protection. Curr Eye Res. 1988 Nov;7(11):1051–1061. doi: 10.3109/02713688809001875. [DOI] [PubMed] [Google Scholar]
- Fung-Leung W. P., Schilham M. W., Rahemtulla A., Kündig T. M., Vollenweider M., Potter J., van Ewijk W., Mak T. W. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991 May 3;65(3):443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Bahri S., Nesburn A. B., Wechsler S. L. Protection against herpes simplex virus-induced eye disease after vaccination with seven individually expressed herpes simplex virus 1 glycoproteins. Invest Ophthalmol Vis Sci. 1995 Jun;36(7):1352–1360. [PubMed] [Google Scholar]
- Ghiasi H., Cai S., Nesburn A. B., Wechsler S. L. MHC-II but not MHC-I responses are required for vaccine-induced protection against ocular challenge with HSV-1. Curr Eye Res. 1997 Nov;16(11):1152–1158. doi: 10.1076/ceyr.16.11.1152.5104. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Cai S., Slanina S., Nesburn A. B., Wechsler S. L. Nonneutralizing antibody against the glycoprotein K of herpes simplex virus type-1 exacerbates herpes simplex virus type-1-induced corneal scarring in various virus-mouse strain combinations. Invest Ophthalmol Vis Sci. 1997 May;38(6):1213–1221. [PubMed] [Google Scholar]
- Ghiasi H., Kaiwar R., Nesburn A. B., Slanina S., Wechsler S. L. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J Virol. 1994 Apr;68(4):2118–2126. doi: 10.1128/jvi.68.4.2118-2126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi H., Roopenian D. C., Slanina S., Cai S., Nesburn A. B., Wechsler S. L. The importance of MHC-I and MHC-II responses in vaccine efficacy against lethal herpes simplex virus type 1 challenge. Immunology. 1997 Jul;91(3):430–435. doi: 10.1046/j.1365-2567.1997.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks R. L., Epstein R. J., Tumpey T. The effect of cellular immune tolerance to HSV-1 antigens on the immunopathology of HSV-1 keratitis. Invest Ophthalmol Vis Sci. 1989 Jan;30(1):105–115. [PubMed] [Google Scholar]
- Hendricks R. L., Epstein R. J., Tumpey T. The effect of cellular immune tolerance to HSV-1 antigens on the immunopathology of HSV-1 keratitis. Invest Ophthalmol Vis Sci. 1989 Jan;30(1):105–115. [PubMed] [Google Scholar]
- Hendricks R. L., Janowicz M., Tumpey T. M. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J Immunol. 1992 Apr 15;148(8):2522–2529. [PubMed] [Google Scholar]
- Hendricks R. L., Tumpey T. M. Contribution of virus and immune factors to herpes simplex virus type I-induced corneal pathology. Invest Ophthalmol Vis Sci. 1990 Oct;31(10):1929–1939. [PubMed] [Google Scholar]
- Igietseme J. U., Streilein J. W., Miranda F., Feinerman S. J., Atherton S. S. Mechanisms of protection against herpes simplex virus type 1-induced retinal necrosis by in vitro-activated T lymphocytes. J Virol. 1991 Feb;65(2):763–768. doi: 10.1128/jvi.65.2.763-768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaian M. T., Leite-Morris K. A., Biron C. A. The role of CD4+ cells in sustaining lymphocyte proliferation during lymphocytic choriomeningitis virus infection. J Immunol. 1991 Mar 15;146(6):1955–1963. [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Hengartner H., Zinkernagel R. M. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994 Dec;24(12):3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Reiner S. L., Hatam F., Littman D. R., Killeen N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science. 1993 Sep 10;261(5127):1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- Maggs D. J., Chang E., Nasisse M. P., Mitchell W. J. Persistence of herpes simplex virus type 1 DNA in chronic conjunctival and eyelid lesions of mice. J Virol. 1998 Nov;72(11):9166–9172. doi: 10.1128/jvi.72.11.9166-9172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matechak E. O., Killeen N., Hedrick S. M., Fowlkes B. J. MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity. 1996 Apr;4(4):337–347. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- Mercadal C. M., Bouley D. M., DeStephano D., Rouse B. T. Herpetic stromal keratitis in the reconstituted scid mouse model. J Virol. 1993 Jun;67(6):3404–3408. doi: 10.1128/jvi.67.6.3404-3408.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadal C. M., Martin S., Rouse B. T. Apparent requirement for CD4+ T cells in primary anti-herpes simplex virus cytotoxic T-lymphocyte induction can be overcome by optimal antigen presentation. Viral Immunol. 1991 Fall;4(3):177–186. doi: 10.1089/vim.1991.4.177. [DOI] [PubMed] [Google Scholar]
- Metcalf J. F., Hamilton D. S., Reichert R. W. Herpetic keratitis in athymic (nude) mice. Infect Immun. 1979 Dec;26(3):1164–1171. doi: 10.1128/iai.26.3.1164-1171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa H., Sakuma S., Mohri S., Mori R., Watanabe T. Herpes simplex virus type 1 infection in mice with severe combined immunodeficiency (SCID). Arch Virol. 1988;103(1-2):73–82. doi: 10.1007/BF01319810. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Dierich A., Benoist C., Mathis D. LAG-3 is not responsible for selecting T helper cells in CD4-deficient mice. Int Immunol. 1996 May;8(5):725–729. doi: 10.1093/intimm/8.5.725. [DOI] [PubMed] [Google Scholar]
- Newell C. K., Martin S., Sendele D., Mercadal C. M., Rouse B. T. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989 Feb;63(2):769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell C. K., Sendele D., Rouse B. T. Effects of CD4+ and CD8+ T-lymphocyte depletion on the induction and expression of herpes simplex stromal keratitis. Reg Immunol. 1989 Nov-Dec;2(6):366–369. [PubMed] [Google Scholar]
- Pepose J. S., Whittum-Hudson J. A. An immunogenetic analysis of resistance to herpes simplex virus retinitis in inbred strains of mice. Invest Ophthalmol Vis Sci. 1987 Sep;28(9):1549–1552. [PubMed] [Google Scholar]
- Rahemtulla A., Kündig T. M., Narendran A., Bachmann M. F., Julius M., Paige C. J., Ohashi P. S., Zinkernagel R. M., Mak T. W. Class II major histocompatibility complex-restricted T cell function in CD4-deficient mice. Eur J Immunol. 1994 Sep;24(9):2213–2218. doi: 10.1002/eji.1830240942. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Nasisse M. P., Larsen H. S., Rouse B. T. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 1984 Aug;25(8):938–944. [PubMed] [Google Scholar]
- Sakai Y., Minagawa H., Ishibashi T., Inomata H., Mori R. Stromal keratitis induced by a unique clinical isolate of herpes simplex virus type 1. Ophthalmologica. 1994;208(3):157–160. doi: 10.1159/000310474. [DOI] [PubMed] [Google Scholar]
- Staats H. F., Oakes J. E., Lausch R. N. Anti-glycoprotein D monoclonal antibody protects against herpes simplex virus type 1-induced diseases in mice functionally depleted of selected T-cell subsets or asialo GM1+ cells. J Virol. 1991 Nov;65(11):6008–6014. doi: 10.1128/jvi.65.11.6008-6014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J., Rouse B. T. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16(4):375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- Tullo A. B., Shimeld C., Blyth W. A., Hill T. J., Easty D. L. Ocular infection with herpes simplex virus in nonimmune and immune mice. Arch Ophthalmol. 1983 Jun;101(6):961–964. doi: 10.1001/archopht.1983.01040010961023. [DOI] [PubMed] [Google Scholar]
- Xie L. X., Gebhardt B. M., Kaufman H. E. [An investigation of HSV-1 antigen in human corneas with recurrent HSK]. Zhonghua Yan Ke Za Zhi. 1991 Mar;27(2):109–111. [PubMed] [Google Scholar]
- Zinkernagel R. M. Allgemeine immunologische Grundlagen und ein Beispiel einer virusbedingten Immunsuppression. Verh Dtsch Ges Pathol. 1991;75:1–3. [PubMed] [Google Scholar]


