Abstract
AIM—To determine whether there are differences in the lymphocytic cell infiltrate present in affected extraocular muscles (EOM) during early and late stages of thyroid associated ophthalmopathy (TAO). METHODS—17 biopsies of affected EOMs were collected from two groups of TAO patients (n=14): the first of five patients with early, active TAO, and the second of nine patients with late, inactive TAO. The control group was of EOM biopsies taken from 14 non-TAO patients undergoing squint surgery. Immunohistochemical analysis was undertaken using the relevant monoclonal antibodies and an avidin-biotin system and the three groups compared. RESULTS—Both CD4+ and CD8+ T cells were found in the cellular infiltrate in early, active TAO specimens which were much less evident either in late, inactive stage disease or in control tissue. There was also a significant increase in both CD45RO+ and CD45RB+ cells and macrophages in early TAO compared with the others. Increased expression of HLA-DR antigen by interstitial cells including fibroblasts was detected in both early and late disease but the EOM fibres remained morphologically intact and did not express MHC class II antigens at any time. CONCLUSION—These results demonstrate that T cells are only significantly present in early disease but increased HLA-DR antigen expression on fibroblasts is observed at all stages. This suggests that T cells are much more involved in the early than the later stages of the disease process and that early activation of fibroblasts occurs. Early intervention with immunosuppressive therapy to downregulate cytokine production by T cells may significantly influence the sequelae caused by EOM fibrosis.
Full Text
The Full Text of this article is available as a PDF (237.3 KB).
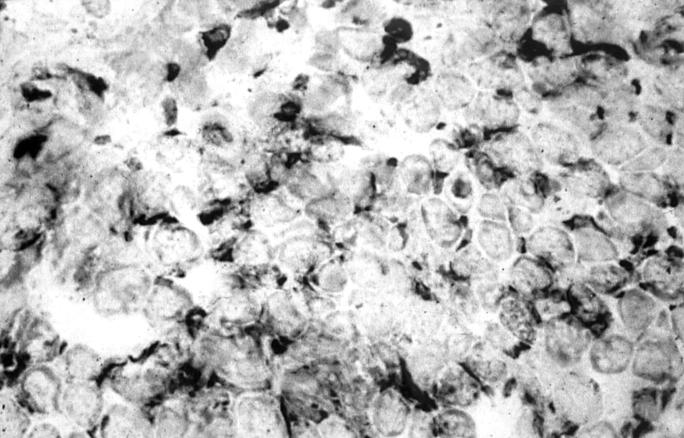
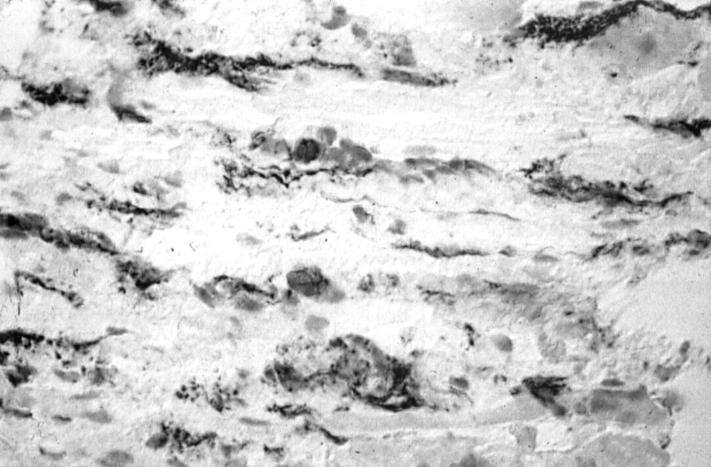
Figure 1 .
Light micrograph of a transversely cut extraocular muscle tissue from a patient with early TAO. Immunoperoxidase staining for CD45R0 on a cryostat section. Positive staining is seen as a black precipitate (×476).
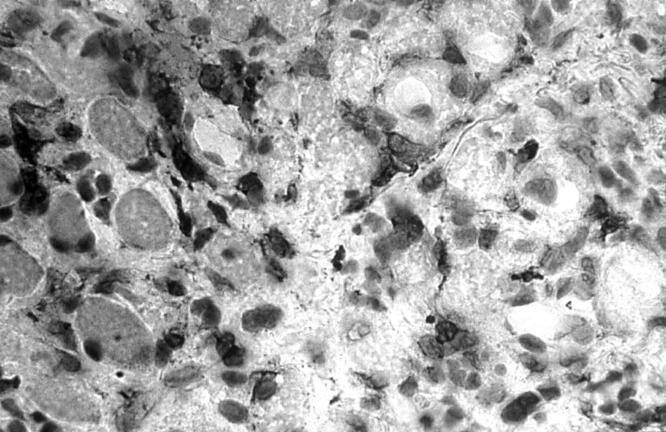
Figure 2 .
Light micrograph of a transversely cut extraocular muscle tissue from a patient with late, inactive TAO. Immunoperoxidase staining for CD45R0 on a cryostat section. Positive staining is seen as a black precipitate (×476).
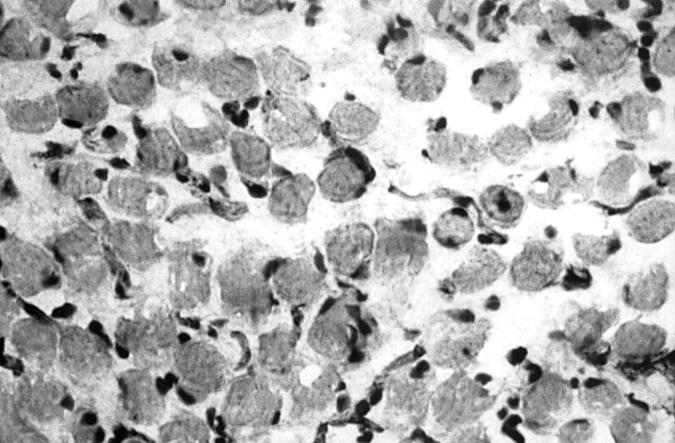
Figure 3 .
Light micrograph of immunoperoxidase staining for macrophages on a cryostat section of intraocular muscle tissue from a patient with early, active TAO (black precipitate) (×476).
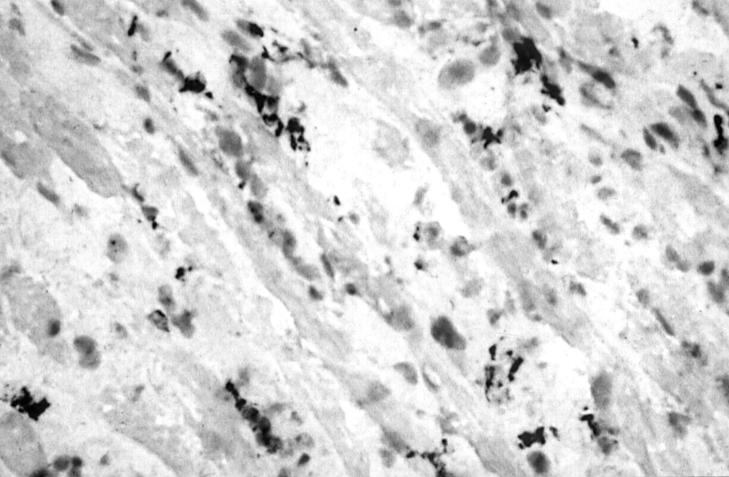
Figure 4 .
Light micrograph of immunoperoxidase staining for macrophages on a cryostat section of extraocular muscle tissue from a patient with late, inactive TAO (black precipitate) (×476).
Figure 5 .
Light micrograph of immunoperoxidase staining for HLA class II (DR) antigen on a cryostat section of extraocular muscle tissue from a patient with early, active TAO. Positively stained cells and their processes are located in the endomysium and perimysium (black precipitate) (×476).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnasco M., Venuti D., Prigione I., Torre G. C., Ferrini S., Canonica G. W. Graves' disease: phenotypic and functional analysis at the clonal level of the T-cell repertoire in peripheral blood and in thyroid. Clin Immunol Immunopathol. 1988 May;47(2):230–239. doi: 10.1016/0090-1229(88)90075-x. [DOI] [PubMed] [Google Scholar]
- Char D. H. Thyroid eye disease. Br J Ophthalmol. 1996 Oct;80(10):922–926. doi: 10.1136/bjo.80.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzmann D. R., Donaldson S. S., Kriss J. P. Appearance of Graves' disease on orbital computed tomography. J Comput Assist Tomogr. 1979 Dec;3(6):815–819. [PubMed] [Google Scholar]
- Fells P. Thyroid-associated eye disease: clinical management. Lancet. 1991 Jul 6;338(8758):29–32. doi: 10.1016/0140-6736(91)90014-g. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E. Involvement of the orbital fibroblast and TSH receptor in the pathogenesis of Graves' ophthalmopathy. Thyroid. 1995 Aug;5(4):331–340. doi: 10.1089/thy.1995.5.331. [DOI] [PubMed] [Google Scholar]
- Hiromatsu Y., Tanaka K., Ishisaka N., Kamachi J., Kuroki T., Hoshino T., Inoue Y., Wall J. R., Nonaka K. Human histocompatibility leukocyte antigen-DR and heat shock protein-70 expression in eye muscle tissue in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1995 Feb;80(2):685–691. doi: 10.1210/jcem.80.2.7531718. [DOI] [PubMed] [Google Scholar]
- Hufnagel T. J., Hickey W. F., Cobbs W. H., Jakobiec F. A., Iwamoto T., Eagle R. C. Immunohistochemical and ultrastructural studies on the exenterated orbital tissues of a patient with Graves' disease. Ophthalmology. 1984 Nov;91(11):1411–1419. doi: 10.1016/s0161-6420(84)34152-5. [DOI] [PubMed] [Google Scholar]
- Jacobson D. H., Gorman C. A. Endocrine ophthalmopathy: current ideas concerning etiology, pathogenesis, and treatment. Endocr Rev. 1984 Spring;5(2):200–220. doi: 10.1210/edrv-5-2-200. [DOI] [PubMed] [Google Scholar]
- Kroll A. J., Kuwabara T. Dysthyroid ocular myopathy. Anatomy, histology, and electron microscopy. Arch Ophthalmol. 1966 Aug;76(2):244–247. doi: 10.1001/archopht.1966.03850010246017. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Martino L. J., Wilcox B. D., Davis F. B., Gordinier J. K., Davis P. J. Potentiation by thyroid hormone of human IFN-gamma-induced HLA-DR expression. J Immunol. 1998 Jul 15;161(2):843–849. [PubMed] [Google Scholar]
- Lombardi G., Arnold K., Uren J., Marelli-Berg F., Hargreaves R., Imami N., Weetman A., Lechler R. Antigen presentation by interferon-gamma-treated thyroid follicular cells inhibits interleukin-2 (IL-2) and supports IL-4 production by B7-dependent human T cells. Eur J Immunol. 1997 Jan;27(1):62–71. doi: 10.1002/eji.1830270110. [DOI] [PubMed] [Google Scholar]
- Ludgate M., Crisp M., Lane C., Costagliola S., Vassart G., Weetman A., Daunerie C., Many M. C. The thyrotropin receptor in thyroid eye disease. Thyroid. 1998 May;8(5):411–413. doi: 10.1089/thy.1998.8.411. [DOI] [PubMed] [Google Scholar]
- Pappa A., Calder V., Ajjan R., Fells P., Ludgate M., Weetman A. P., Lightman S. Analysis of extraocular muscle-infiltrating T cells in thyroid-associated ophthalmopathy (TAO). Clin Exp Immunol. 1997 Aug;109(2):362–369. doi: 10.1046/j.1365-2249.1997.4491347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa A., Calder V., Fells P., Lightman S. Adhesion molecule expression in vivo on extraocular muscles (EOM) in thyroid-associated ophthalmopathy (TAO). Clin Exp Immunol. 1997 May;108(2):309–313. doi: 10.1046/j.1365-2249.1997.3621258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perros P., Kendall-Taylor P. Pathogenetic mechanisms in thyroid-associated ophthalmopathy. J Intern Med. 1992 Mar;231(3):205–211. doi: 10.1111/j.1365-2796.1992.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Perros P., Kendall-Taylor P. Thyroid-associated ophthalmopathy: pathogenesis and clinical management. Baillieres Clin Endocrinol Metab. 1995 Jan;9(1):115–135. doi: 10.1016/s0950-351x(95)80867-1. [DOI] [PubMed] [Google Scholar]
- Sempowski G. D., Rozenblit J., Smith T. J., Phipps R. P. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. 1998 Mar;274(3 Pt 1):C707–C714. doi: 10.1152/ajpcell.1998.274.3.C707. [DOI] [PubMed] [Google Scholar]
- Sergott R. C., Glaser J. S. Graves' ophthalmopathy. A clinical and immunologic review. Surv Ophthalmol. 1981 Jul-Aug;26(1):1–21. doi: 10.1016/0039-6257(81)90120-x. [DOI] [PubMed] [Google Scholar]
- Solomon D. H., Chopra I. J., Chopra U., Smith F. J. Identification of subgroups of euthyroid graves's ophthalmopathy. N Engl J Med. 1977 Jan 27;296(4):181–186. doi: 10.1056/NEJM197701272960401. [DOI] [PubMed] [Google Scholar]
- Tallstedt L., Norberg R. Immunohistochemical staining of normal and Graves' extraocular muscle. Invest Ophthalmol Vis Sci. 1988 Feb;29(2):175–184. [PubMed] [Google Scholar]
- Wall J. R., Salvi M., Bernard N. F., Boucher A., Haegert D. Thyroid-associated ophthalmopathy--a model for the association of organ-specific autoimmune disorders. Immunol Today. 1991 May;12(5):150–153. doi: 10.1016/S0167-5699(05)80044-1. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., Cohen S., Gatter K. C., Fells P., Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves' ophthalmopathy. Clin Exp Immunol. 1989 Feb;75(2):222–227. [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P. Thyroid-associated eye disease: pathophysiology. Lancet. 1991 Jul 6;338(8758):25–28. doi: 10.1016/0140-6736(91)90013-f. [DOI] [PubMed] [Google Scholar]
- Werner S. C., Coleman D. J., Franzen L. A. Ultrasonographic evidence of a consistent orbital involvement in Graves's disease. N Engl J Med. 1974 Jun 27;290(26):1447–1450. doi: 10.1056/NEJM197406272902602. [DOI] [PubMed] [Google Scholar]
- Werner S. C. Modification of the classification of the eye changes of Graves' disease. Am J Ophthalmol. 1977 May;83(5):725–727. doi: 10.1016/0002-9394(77)90140-4. [DOI] [PubMed] [Google Scholar]
- van der Gaag R., Vernimmen R., Fiebelkorn N., van Dierendonck M. C., Kijlstra A. Graves' ophthalmopathy: what is the evidence for extraocular muscle specific autoantibodies. Int Ophthalmol. 1990 Jan;14(1):25–30. doi: 10.1007/BF00131165. [DOI] [PubMed] [Google Scholar]