Abstract
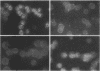
The effect of 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil (BV-araU), a new antiviral drug, on Epstein-Barr virus (EBV) was studied and compared with those of E-5-(2-bromovinyl)-2'-deoxyuridine (BVdU) and acyclovir (ACV). BV-araU effectively inhibited EBV replication both in superinfected Raji cells and in virus producer P3HR-1(LS) cells, as determined by density gradient centrifugation, in situ cytohybridization with an EBV DNA probe, and cRNA-DNA hybridization. The 50% effective doses for viral DNA replication were 0.26, 0.06, and 0.3 microM for BV-araU, BVdU, and ACV, respectively. The relative efficacy on the basis of the in vitro therapeutic index was BVdU (6,500) greater than BV-araU (1,500) greater than ACV (850). Synthesis of EBV-induced polypeptides with molecular weights of 145,000 and 140,000 was inhibited by these drugs. Kinetic analysis of reversibility of inhibition of EBV DNA replication after removal of the drugs indicated that BV-araU, like BVdU, has a more prolonged inhibitory effect than ACV. These results indicate that the 2' OH group in the arabinosyl configuration of BV-araU results in marked reduction in anti-EBV activity while slightly diminishing cytotoxicity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp L. M., Serling B. L., Kelsey J. E., Biron K. K., Collins P., Selway J., Lin J. C., Schaeffer H. J. Effect of acyclic pyrimidines related to 9-[(1,3-dihydroxy-2-propoxy)methyl]guanine on herpesviruses. J Med Chem. 1988 Jan;31(1):144–149. doi: 10.1021/jm00396a021. [DOI] [PubMed] [Google Scholar]
- Clough W. Deoxyribonuclease activity found in Epstein--Barr virus producing lymphoblastoid cells. Biochemistry. 1979 Oct 16;18(21):4517–4521. doi: 10.1021/bi00588a009. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., De Somer P., Barr P. J., Jones A. S., Walker R. T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):9–17. doi: 10.1093/jac/12.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., DeClercq E., Pagano J. S. Novel acyclic adenosine analogs inhibit Epstein-Barr virus replication. Antimicrob Agents Chemother. 1987 Sep;31(9):1431–1433. doi: 10.1128/aac.31.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Nelson D. J., Lambe C. U., Choi E. I. Metabolic activation of 9([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine in human lymphoblastoid cell lines infected with Epstein-Barr virus. J Virol. 1986 Nov;60(2):569–573. doi: 10.1128/jvi.60.2.569-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Pagano J. S. Synthesis of chromosomal proteins and Epstein-Barr virus DNA in activated Burkitt somatic cell hybrids. Virology. 1980 Oct 15;106(1):50–58. doi: 10.1016/0042-6822(80)90220-2. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Raab-Traub N. Two strains of Epstein-Barr virus (B95-8 and a P3HR-1 subclone) that lack defective genomes induce early antigen and cause abortive infection of Raji cells. J Virol. 1987 Jun;61(6):1985–1991. doi: 10.1128/jvi.61.6.1985-1991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Shaw J. E., Smith M. C., Pagano J. S. Effect of 12-O-tetradecanoyl-phorbol-13-acetate on the replication of Epstein-Barr virus. I. Characterization of viral DNA. Virology. 1979 Nov;99(1):183–187. doi: 10.1016/0042-6822(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Cheng Y. C., Pagano J. S. Epstein-Barr virus: inhibition of replication by three new drugs. Science. 1983 Aug 5;221(4610):578–579. doi: 10.1126/science.6306771. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Choi E. I., De Clercq E., Verbruggen A., Pagano J. S. Effect of (E)-5-(2-bromovinyl)-2'-deoxyuridine on replication of Epstein-Barr virus in human lymphoblastoid cell lines. Antiviral Res. 1985;Suppl 1:121–126. doi: 10.1016/s0166-3542(85)80018-8. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Activation of latent Epstein-Barr virus genomes: selective stimulation of synthesis of chromosomal proteins by a tumor promoter. J Virol. 1983 Mar;45(3):985–991. doi: 10.1128/jvi.45.3.985-991.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Comparative efficacy and selectivity of some nucleoside analogs against Epstein-Barr virus. Antimicrob Agents Chemother. 1985 Jun;27(6):971–973. doi: 10.1128/aac.27.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Effects of 12-O-tetradecanoyl-phorbol-13-acetate on cell proliferation and Epstein-Barr virus DNA replication. Virology. 1982 Feb;117(1):186–194. doi: 10.1016/0042-6822(82)90518-9. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Prolonged inhibitory effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine against replication of Epstein-Barr virus. J Virol. 1984 Apr;50(1):50–55. doi: 10.1128/jvi.50.1.50-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida H. Comparison of susceptibilities of varicella-zoster virus and herpes simplex viruses to nucleoside analogs. Antimicrob Agents Chemother. 1986 Mar;29(3):524–526. doi: 10.1128/aac.29.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida H., Kuninaka A., Yoshino H. Inhibitory effects of antiherpesviral thymidine analogs against varicella-zoster virus. Antimicrob Agents Chemother. 1982 Feb;21(2):358–361. doi: 10.1128/aac.21.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida H., Sakata S., Kuninaka A., Yoshino H. Antiherpesviral and anticellular effects of 1-beta-D-arabinofuranosyl-E-5-(2-halogenovinyl) uracils. Antimicrob Agents Chemother. 1981 Jul;20(1):47–52. doi: 10.1128/aac.20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander M., Cheng Y. C. Properties of herpes simplex virus type 1 and type 2 DNA polymerase. Biochim Biophys Acta. 1980 Sep 19;609(2):232–245. doi: 10.1016/0005-2787(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Sim I. S., Goodchild J., Meredith D. M., Porter R. A., Raper R. H., Viney J., Wadsworth H. J. Possible molecular basis for antiviral activity of certain 5-substituted deoxyuridines. Antimicrob Agents Chemother. 1983 Mar;23(3):416–421. doi: 10.1128/aac.23.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]