Abstract
AIMS—To assess the potential role of macrophage migration inhibitory factor (MIF) in the pathogenesis of proliferative diabetic retinopathy (PDR). METHODS—MIF levels were assayed in the vitreous and paired serum samples of 73 consecutive patients with PDR (32 eyes) and macular hole or idiopathic epiretinal membrane (controls, 41 eyes). An enzyme linked immunosorbent assay technique was used to determine the concentrations of MIF. RESULTS—The median vitreous level of MIF was 11.93 ng/ml (range 4.16-103.85) in the patients with PDR, and 1.79 ng/ml (undetectable-8.93) in the controls. Vitreous levels in eyes with PDR were significantly greater than those in the controls (p<0.0001). Vitreous levels were significantly higher than serum levels in eyes with PDR (p=0.0026). MIF levels were significantly higher in the vitreous of PDR patients with severe fibrous proliferation than in those with slight proliferation (p<0.05). CONCLUSION—The results indicate increased levels of MIF in the vitreous of patients with PDR and a significant association between MIF levels and grades of fibrous proliferation, suggesting the possibility that MIF may play a part in the development of the proliferative phase of PDR.
Full Text
The Full Text of this article is available as a PDF (118.4 KB).
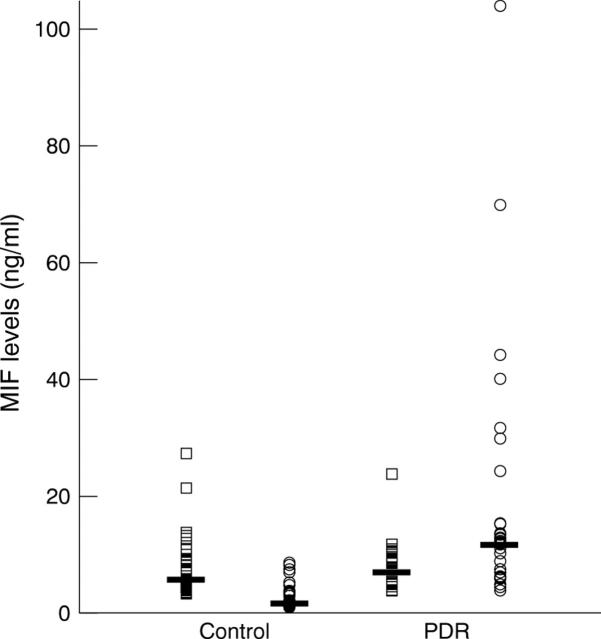
Figure 1 .
Macrophage migration inhibitory factor (MIF) levels in the vitreous and paired serum samples from eyes with macular hole or idiopathic epiretinal membrane (controls: n=41) and proliferative diabetic retinopathy (PDR: n=32). Open circles represent vitreous levels and open squares represent serum levels. The horizontal lines indicate the median concentration in each group. The vitreous MIF levels in PDR were significantly greater than levels in the controls (p<0.0001). Vitreous MIF levels were significantly higher than serum MIF levels in PDR (p=0.0026), but significantly lower than serum levels in the controls (p<0.0001).
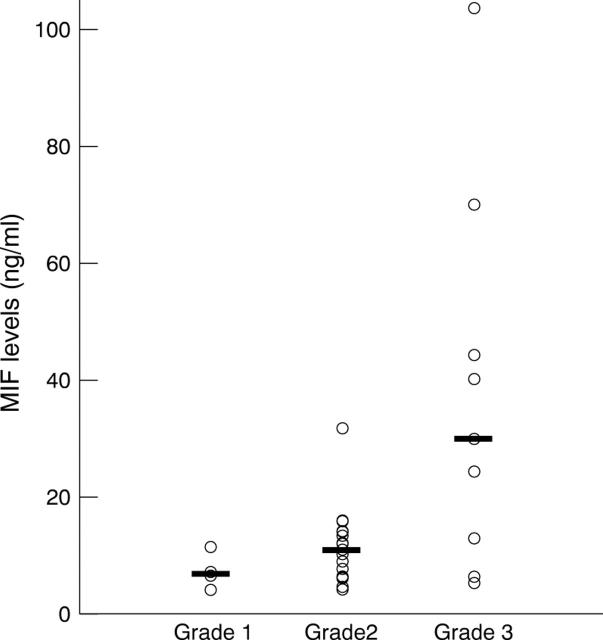
Figure 2 .
Macrophage migration inhibitory factor (MIF) levels in the vitreous samples from eyes with grade 1 (n=4), grade 2 (n=19), and grade 3 (n=9) of fibrous proliferation in proliferative diabetic retinopathy (PDR). The horizontal lines indicate the median concentration in each group. Vitreous MIF levels were significantly greater in grade 3 than in grades 1 or 2 (p=0.0076, p=0.0009, respectively).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu el-Asrar A. M., Van Damme J., Put W., Veckeneer M., Dralands L., Billiau A., Missotten L. Monocyte chemotactic protein-1 in proliferative vitreoretinal disorders. Am J Ophthalmol. 1997 May;123(5):599–606. doi: 10.1016/s0002-9394(14)71072-4. [DOI] [PubMed] [Google Scholar]
- Bacher M., Metz C. N., Calandra T., Mayer K., Chesney J., Lohoff M., Gemsa D., Donnelly T., Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin C., Fredj-Reygrobellet D., Gordon W. C., Baudouin F., Peyman G., Lapalus P., Gastaud P., Bazan N. G. Immunohistologic study of epiretinal membranes in proliferative vitreoretinopathy. Am J Ophthalmol. 1990 Dec 15;110(6):593–598. doi: 10.1016/s0002-9394(14)77054-0. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Mitchell R. A., Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994 Jun 1;179(6):1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capeans C., De Rojas M. V., Lojo S., Salorio M. S. C-C chemokines in the vitreous of patients with proliferative vitreoretinopathy and proliferative diabetic retinopathy. Retina. 1998;18(6):546–550. [PubMed] [Google Scholar]
- Elner S. G., Elner V. M., Jaffe G. J., Stuart A., Kunkel S. L., Strieter R. M. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995 Nov;14(11):1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- Esser P., Heimann K., Wiedemann P. Macrophages in proliferative vitreoretinopathy and proliferative diabetic retinopathy: differentiation of subpopulations. Br J Ophthalmol. 1993 Nov;77(11):731–733. doi: 10.1136/bjo.77.11.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase K., Ikeda T., Sotozono C., Nishida K., Sawa H., Kinoshita S. Transforming growth factor beta2 in the vitreous in proliferative diabetic retinopathy. Arch Ophthalmol. 1998 Jun;116(6):738–741. doi: 10.1001/archopht.116.6.738. [DOI] [PubMed] [Google Scholar]
- Limb G. A., Alam A., Earley O., Green W., Chignell A. H., Dumonde D. C. Distribution of cytokine proteins within epiretinal membranes in proliferative vitreoretinopathy. Curr Eye Res. 1994 Nov;13(11):791–798. doi: 10.3109/02713689409025133. [DOI] [PubMed] [Google Scholar]
- Matsuda A., Tagawa Y., Yoshida K., Matsuda H., Nishihira J. Expression of macrophage migration inhibitory factor in rat retina and its immunohistochemical localization. J Neuroimmunol. 1997 Jul;77(1):85–90. doi: 10.1016/s0165-5728(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihira J., Kuriyama T., Nishino H., Ishibashi T., Sakai M., Nishi S. Purification and characterization of human macrophage migration inhibitory factor: evidence for specific binding to glutathione and formation of subunit structure. Biochem Mol Biol Int. 1993 Dec;31(5):841–850. [PubMed] [Google Scholar]
- Rossi A. G., Haslett C., Hirani N., Greening A. P., Rahman I., Metz C. N., Bucala R., Donnelly S. C. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. J Clin Invest. 1998 Jun 15;101(12):2869–2874. doi: 10.1172/JCI1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Le-Ruppert K. C. Activated T lymphocytes in epiretinal membranes from eyes of patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1995 Jan;233(1):21–25. doi: 10.1007/BF00177781. [DOI] [PubMed] [Google Scholar]
- Tang S., Scheiffarth O. F., Thurau S. R., Wildner G. Cells of the immune system and their cytokines in epiretinal membranes and in the vitreous of patients with proliferative diabetic retinopathy. Ophthalmic Res. 1993;25(3):177–185. doi: 10.1159/000267287. [DOI] [PubMed] [Google Scholar]