Abstract
IL-1β and its endogenous receptor antagonist (IL-1Ra) are rapidly induced by seizures in the rodent hippocampus. Exogenously applied IL-1β prolongs seizures in an IL-1R type I-mediated manner. This effect depends on N-methyl-d-aspartate receptor activation. We report here that intrahippocampal application of recombinant IL-1Ra or its selective endogenous overexpression in astrocytes under the control of glial acidic fibrillary protein promoter potently inhibits motor and electroencephalographic seizures induced by bicuculline methiodide in mice. Accordingly, transgenic mice show a reduced seizure-related c-fos mRNA expression in various forebrain areas compared with their wild-type littermates. Recombinant IL-1Ra was ineffective in mice deficient in IL-1R type I, having per se a delayed onset to generalized convulsions. These results demonstrate that IL-1Ra mediates potent anticonvulsant effects acting on IL-1R type I and suggest that the balance between brain IL-1β and IL-1Ra represents a crucial mechanism to control seizure generalization.
Proinflammatory cytokines are important soluble mediators of cellular communication in both physiologic and pathophysiologic states. There is accumulating evidence that among the proinflammatory cytokines, IL-1β plays a significant role as effector of both the clinical and pathological features of various central nervous system diseases (1, 2).
IL-1β appears to be involved in neuronal network excitability because it affects the turnover and release of various neurotransmitters (1) and the expression of neuropeptides and neurotrophic factors (3–5) and alters synaptic transmission and ionic currents (6–9) in several rodent forebrain regions.
Convulsant stimuli increase the production of IL-1β, its naturally occurring receptor antagonist (IL-1Ra), and IL-1R type I and II predominantly in glia in rodent central nervous system within hours of seizure induction (10–15).
We recently showed that IL-1β prolongs hippocampal electroencephalographic (EEG) seizures in a N-methyl-d-aspartate receptor-dependent manner, and this action was blocked by IL-1Ra (14).
In this study, we investigated whether IL-1Ra has anticonvulsant properties in rodents. We found that intracerebral application of recombinant IL-1Ra or its endogenous overexpression in astrocytes potently inhibited behavioral and EEG seizures induced by bicuculline methiodide in mice. This effect was mediated specifically by IL-1R type I, because IL-1Ra was ineffective in knockout mice deficient in these receptors.
Thus, the functional interaction between brain-born IL-1β and IL-1Ra during seizures, (i) may play a critical role in the physiopathological functions of IL-1β, and (ii) may significantly affect the maintenance and spread of seizures.
Materials and Methods
Animals.
Procedures involving animals and their care were conducted in conformity with institutional guidelines in compliance with national and international laws and policies (4D. L. N. 116, Gazzetta Ufficiale, supplement 40, 18–2-1992 and European Economic Community Council Directive 86/609, OJ L 358, 1, issued on 12 December 1987; National Institutes of Health Guide for the Care and Use of Laboratory Animals, U. S. National Research Council, 1996).
B6/CBA and 129/SV PasIco adult male mice (25–30 g; Department of Neurochemistry and Neurotoxicology, University of Stockholm, Stockholm, Sweden) were used. The wild-type mice used as controls of IL-1Ra overexpressing mice (GILRA2) and IL-1R-type I-deficient mice were littermates of the corresponding strain (Tables 1 and 2).
Table 1.
Decreased susceptibility to bicuculline methiodide-induced seizures in transgenic mice overexpressing the human soluble form of IL-1Ra in astrocytes (GILRA2)
| No mice with
seizures
|
Onset, min
|
Duration, min
|
||||
|---|---|---|---|---|---|---|
| Clonic | Tonic | Clonus | Tonus | Clonus | Tonus | |
| Wild type | 11/11 | 11/11 | 2.9 ± 0.4 | 6.9 ± 1.3 | 68.9 ± 4.2 | 2.9 ± 0.7 |
| GILRA2 | 7/7 | 4/7* | 2.5 ± 0.5 | 44.7 ± 16† | 22.4 ± 7.9† | 0.7 ± 0.3‡ |
Data are the mean ± S.E.M. Fractions represent the number of B6/CBA mice showing clonic or tonic seizures on the total number of mice. Bicuculline methiodide was injected at a dose of 0.24 nmol in 0.5 μl unilaterally in the dorsal hippocampus. The onset of tonus was reckoned as 90 min (total observation time) in the mice not showing this behavior (n = 3). A significantly lower number of mice overexpressing IL-1Ra (GILRA2) showed tonic convulsions after bicuculline. These mice showed a significant reduction in the duration of generalized seizures.
, P < 0.05,
, P < 0.01 by Student's t test; *, P < 0.05 by Fisher's test vs. wild-type littermate controls injected with bicuculline (wild type). Representative EEG recordings of ictal and interictal activity are shown in Fig. 3.
Table 2.
Susceptibility to bicuculline methiodide-induced seizures in mice deficient in IL-1 receptor-type I: effect of intrahippocampal infusion of recombinant IL-1Ra
| No mice with seizures
|
Onset,
min
|
Duration, min
|
||||
|---|---|---|---|---|---|---|
| Clonic | Tonic | Clonus | Tonus | Clonus | Tonus | |
| Wild type | 15/15 | 15/15 | 2.6 ± 0.2 | 5.8 ± 0.6 | 118.2 ± 7.3 | 4.3 ± 0.7 |
| Wild type + IL-1Ra | 15/15 | 15/15 | 5.6 ± 0.5* | 12.1 ± 1.3* | 102.4 ± 6.8 | 1.1 ± 0.1* |
| Knockout | 10/10 | 10/10 | 3.3 ± 0.3† | 7.5 ± 0.6* | 108.0 ± 10.4 | 2.7 ± 0.3 |
| Knockout + IL-1Ra | 10/10 | 9/10 | 3.7 ± 0.8† | 7.4 ± 0.7* | 134.0 ± 11.0 | 3.3 ± 0.5 |
Data are the mean ± S.E.M. Fractions represent the number of 129/SV PasIco mice showing clonic or tonic seizures on the total number of mice. Bicuculline methiodide was injected at a dose of 0.97 nmol in 0.5 μl unilaterally in the dorsal hippocampus. IL-1Ra (0.3 nmol in 0.5 μl) was injected at the same site as bicuculline, 5 min before the convulsant. Recombinant IL-1Ra significantly delayed the onset of clonic and tonic seizures and reduced the duration of tonic convulsions in wild-type littermate mice, whereas it was ineffective in IL-1R type I-deficient mice. Note the delayed onset of clonic and tonic seizures in IL-1R type I-deficient mice (knockout) compared to wild-type littermate mice. Wild-type and knockout mice were injected with heat-inactivated cytokine before bicuculline.
, P < 0.05; *, P < 0.01 vs. wild type by Tukey's test.
B6/CBA F1 mice (Charles River Breeding Laboratories) were used in some experiments (Figs. 1 and 2).
Figure 1.
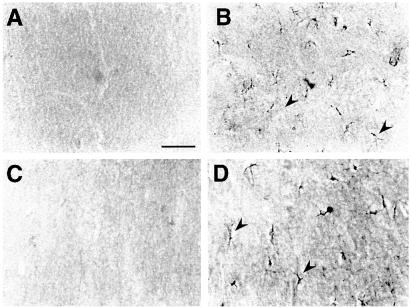
High-magnification photomicrographs showing IL-1β (A and B) and IL-1Ra (C and D) immunoreactivity in the hippocampus of representative B6/CBA F1 mice locally injected with 0.08 nmol bicuculline methiodide (B and D) compared with vehicle-injected controls (A and C). A–D depict corresponding areas of the molecular layer of the dentate gyrus in the injected hippocampus. IL-1β (B) and IL-1Ra (D) staining was enhanced in cells with glial morphology 2 and 4 h after bicuculline injection respectively (arrowheads). No staining was apparent in vehicle-injected mice (A and C) or in mice receiving heat-inactivated cytokines (not shown). A similar immunocytochemical pattern of induction was observed after bicuculline-induced seizures in wild-type SV/129 littermate mice of IL-1R-type I knockout mice (see Table 2) compared with their respective vehicle-injected controls. (Bar = 100 μm.)
Figure 2.
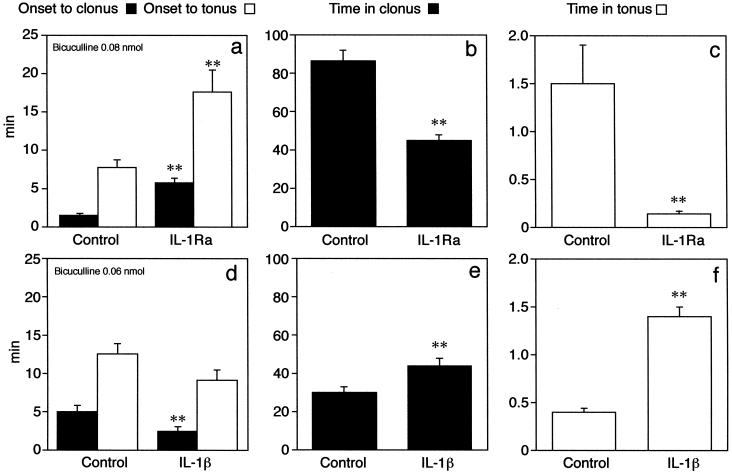
Effects of unilateral intrahippocampal injection of 0.3 nmol IL-1Ra (a–c) or 3 pmol IL-1β (d–f) on motor seizure response of B6/CBA F1 mice to bicuculline methiodide. Data are the mean ± S.E.M. This strain of mice was the same used for engineering transgenic mice overexpressing the human secretable form of IL-1Ra. The anticonvulsant effect of IL-1Ra is denoted by the significant delay in the onset of motor seizures (a) and the reduction in their duration (b and c). All 13 mice in the control group showed both clonic and tonic seizures, whereas 2 mice of 14 did not show tonic seizures after IL-1Ra. The proconvulsant effect of IL-1β is depicted by earlier onset of clonic seizures (d) and by the increase in the duration of motor seizures (e and f). All nine mice in each experimental group showed both clonic and tonic seizures. The proconvulsant effect of IL-1β was assessed by using a submaximal convulsant dose of bicuculline (0.06 nmol) compared with the dose of 0.08 nmol used for testing the anticonvulsant activity of IL-1Ra. Control mice are injected with the corresponding heat-inactivated cytokine before bicuculline methiodide, and they do not differ from B6/CBA F1 mice (n = 6) receiving vehicle (sterile saline) before bicuculline methiodide. Time in clonus or in tonus was reckoned by adding the duration of each convulsive episode occurring during the 120-min observation period. **, P < 0.01 by Student's t test vs. respective controls.
The IL-1Ra, overexpressing mouse strain (B6/CBA) was generated and characterized as previously described (16). Briefly, the secretable form of human recombinant IL-1Ra is expressed in astroglia under the glial fibrillary acidic protein promoter, leading to 10- to 23-fold increase of basal IL-1Ra levels in the central nervous system. In our experiments, we used the strain GILRA2 showing an increase of about 15-fold in CSF IL-1Ra. Total brain homogenates contained approximately 50 ng IL-1Ra (16).
IL-1R type I-deficient mice (129/SV) were generated with a deletion of an ≈1-kb region of the IL-1R type I locus, including the exon encoding the signal peptide. These mice are deficient in the expression of IL-1R type I, as previously described (17).
Both strains of genetically modified mice exhibit no fever or IL-6 induction in response to IL-1. These mice are healthy, fertile, and lack any obvious developmental abnormalities.
Intracerebral Injections and EEG Recordings.
For intrahippocampal injections, mice were anaesthetized with Equithesin (1% phenobarbital/4% chloral hydrate, Sigma), and an injection guide cannula was unilaterally positioned on top of the dura. For simultaneous EEG recordings, nichrome-insulated bipolar depth electrodes were implanted in the injected and contralateral hippocampus [coordinated from bregma: (mm) (nose bar 0); AP-1.9; L ± 1.5; 1.5 below dura], and a ground lead was positioned over the nasal sinus (14). Cannula and electrodes were connected to a multipin socket and secured to the skull with acrylic dental cement. Mice were allowed 3–5 days to recover from the surgical procedure before the start of the study.
Drugs.
Bicuculline methiodide was dissolved in PBS (pH 7.4) and unilaterally injected in the dorsal hippocampus (septal pole) in doses ranging between 0.12 and 0.97 nmol in 0.5 μl, depending on the strain and source of mice. The dose of bicuculline was adjusted in the various experiments to induce motor seizure activity of comparable severity in 100% of wild-type mice with ≤20% mortality. This seizure model was chosen to provoke recurrent motor seizures and EEG ictal and interictal activity in the absence of neurodegenerative events. The lack of neurodegeneration was assessed by Fluoro jade and Nissl staining of forebrain sections 48 h to 5 days after bicuculline injection (not shown).
hrIL-1Ra (inhibitory activity in murine thymocyte proliferation assay, 1.7 × 106 units/mg) and hrIL-1β (bioactivity in murine thymocyte stimulation assay, about 3 × 107 units/mg) (D. Boraschi, Dompé, L'Aquila, Italy) were dissolved in sterile saline and injected in 0.5 μl 5 min before bicuculline. Mice injected with corresponding amounts of heat-inactivated cytokines or corresponding vehicle before bicuculline methiodide were used as controls.
All of the pharmacological experiments were carried out between 9:00 a.m. and 1:00 p.m.
Seizure Assessment.
Motor seizures were observed visually by two independent investigators unaware of the identity of the experimental groups. They were quantified in experimental and matched control mice by using the following parameters: (i) the time to onset of the first seizure (either clonic or tonic); (ii) the duration of the clonic and tonic component of seizures; (iii) the number of motor seizures; (iv) the number of mice showing motor seizures. Clonic seizures consisted of a rhythmic contraction of forelimbs and/or hindlimbs and/or the back muscles. A tonic seizure consisted of a rigid extension of the fore- and/or hindlimbs with or without loss of posture. The time of observation was of 120 min.
EEG recordings were carried out in the hippocampus of freely moving mice (14). A 15-min baseline recording was done to assess the spontaneous EEG pattern. Drugs were injected through an injection needle that extended 1.5 mm below the guide cannula to reach the dorsal hippocampus. The EEG recordings were made continuously for at least 90 min after drug injection. Ictal episodes were characterized by high-frequency and/or multispike complexes and/or high-voltage synchronized spikes simultaneously occurring in both hippocampi (Fig. 3A).
Figure 3.
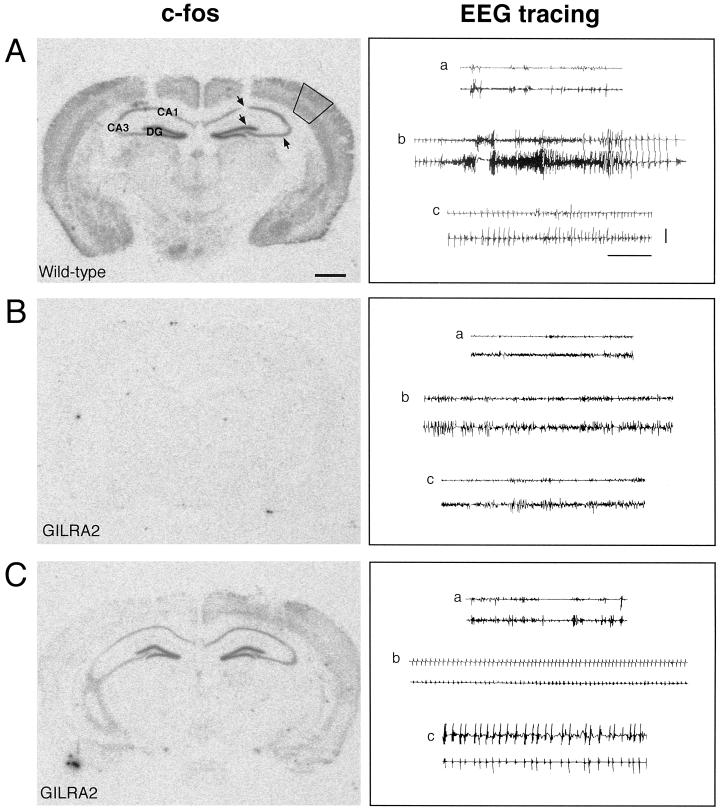
C-fos mRNA expression after intrahippocampal injection of bicuculline methiodide in B6/CBA wild-type and transgenic mice overexpressing IL-1Ra in astrocytes (GILRA2). First column: C-fos was widely and massively expressed in the central nervous system after injection of bicuculline methiodide in wild-type B6/CBA mice (n = 5) (A). In contrast, mice overexpressing IL-1Ra (GILRA2, n = 7) showed no induction (B, n = 4) or considerably milder induction (C, n = 3) in most forebrain areas, particularly in the neocortex and hippocampus. No specific hybridization signal was found in naive mice (not shown). In A, solid lines in the neocortex and arrows in the hippocampus represent the areas where the hybridization signal was quantified (see text). Second column: Representative EEG tracings of the hippocampus in each row depict epileptic activity after injection of bicuculline methiodide in the corresponding wild-type (A) and GILRA2 mice (B and C) (see Table 1 for quantification of motor seizures). (a) Baseline recording before bicuculline injection; (b) ictal activity recorded in wild-type mice (A) was absent in one GILRA2 (B), whereas it was significantly reduced in number and duration in the remaining animals; (c) interictal spiking was interposed between seizures in wild-type mice (A), and it was present throughout the EEG recording in GILRA2 mice (C). Lower and upper traces in each cluster are the injected and contralateral hippocampus, respectively. (Horizontal bar = 10 sec; vertical bar = 100 μV.)
C-fos mRNA Expression.
Mice were killed by decapitation 90 min after bicuculline methiodide injection, and their brains were rapidly frozen into isopentane (−70°C). Coronal sections (20 μm) were cut in a cryotome, mounted on gelatin-coated slides, and stored at −30°C. For in situ hybridization, a synthetic oligonucleotide (Microsynth, Bolgach, Switzerland) complementary to bases 2262–2309 of the mouse c-fos gene was endlabeled with [35S]dATP (1,250 Ci/mmol, NEN) by reaction with terminal deoxynucleotidyl transferase (Boehringer Mannheim) and precipitated with ethanol/sodium chloride. Frozen slides were prehybridized and hybridized as described (18). After the hybridization procedure, the sections were immersed in 70% ethanol, dried, and exposed to β-max films for 24 h to 7 days.
The autoradiograms were digitized and quantified by using a Sony VC44 video camera and a MetaMorph image analysis system (Visitron, Munich, Germany). Optical densities (OD) were calculated from gray values in the frontoparietal cortex and pyramidal and granule neurons of the hippocampus in both hemispheres. Background values were determined in the adjacent white matter and subtracted from OD values. Values were averaged from three to six sections for each mouse. A standard curve was obtained from OD of 14C-microscales (Amersham) and was used for quantification. Arbitrary units were calculated by correcting the OD values for the specific activity of 35S-dATP and for the number of nucleotides incorporated into the oligonucleotide.
Immunocytochemistry.
A separate group of B6/CBA F1 mice were injected with 0.08 nmol bicuculline methiodide or the corresponding vehicle and killed at various times (2, 4, 18, 24 h) after onset of seizures. These experimental animals and their time-matched controls (n = four per group) were transcardially perfused under deep Equithesin anesthesia by using PBS followed by 4% paraformaldehyde as previously described in detail (14). Immunocytochemistry was carried out as described by Vezzani et al. (14) by using 35 μm freely floating coronal brain sections cut with a cryostat at −20°C throughout the septal and temporal pole of the hippocampus. Slices were incubated with the primary antisera at 4°C for 72 h by using a rat monoclonal anti-mouse IL-1β biotin conjugate (6 μg/ml; Biosource International, Camarillo, CA) or a goat anti-mouse IL-1Ra antibody (1.5 μg/ml; R & D Systems). After three 5-min washes in PBS, immunoreactivity was tested by the avidin-biotin-peroxidase technique (Vectastain ABC kit; Vector Laboratories). The sections were then reacted with 0.4 mM 3′-3′-diaminobenzidine (Sigma), washed in PBS, dehydrated, coverslipped, and observed at the light microscope. Control slices were prepared by using the primary antisera preadsorbed with (hr)IL-1β or (hr)IL-1Ra (1 μM, 24 h, 4°C) and by incubating the slices without the primary antisera.
Results
Seizures were induced in mice by intrahippocampal injection of bicuculline methiodide, a selective antagonist of GABAA receptors (19).
By using immunocytochemistry, we found that IL-1β and IL-1Ra are enhanced in the injected hippocampus 2 and 4 h, respectively, after bicuculline-induced seizures in cells with glial morphology (Fig. 1). IL-1Ra immunoreactivity was not observed in the injected hippocampus 2 h after bicuculline injection; however, it was still higher than control after 24 h. IL-1β staining was similar to control level 4 h after seizures (not shown). This is in line with recent findings of increased glial production of inflammatory cytokines (12–15) and IL-1Ra (13,15) in the rat hippocampus in different models of seizures.
Intrahippocampal Injection of Recombinant IL-1β or IL-1Ra.
Preliminary experiments carried out in C57 BL6 adult male mice showed that intracerebral administration of 0.06, 0.3, and 0.6 nmol human recombinant IL-1Ra inhibited bicuculline-induced motor seizures and delayed their time to onset, whereas lower doses were ineffective (data not shown).
Similarly, unilateral injection of 0.3 nmol IL-1Ra in the hippocampus of B6/CBA F1 mice effectively reduced behavioral convulsions induced by a local injection of bicuculline methiodide in the 120-min observation period (Fig. 2 a–c). Thus, the onset time to tonic-clonic seizures was delayed by 2- to 4-fold on average (P < 0.01), and the time spent in clonic and tonic seizures was significantly decreased by 50% and 90%, respectively (P < 0.01). The number of tonic seizures was reduced from 6.0 ± 2.0 in control mice (receiving heat-inactivated IL-1Ra before bicuculline) to 2.0 ± 0.2 (P < 0.05).
Unilateral injection of 3 pmol IL-1β worsened the seizure pattern (Fig. 2 d–f) by significantly reducing the latency to clonus by 52% (P < 0.01) and increasing the time in clonic and tonic seizures by 47% and 250% (P < 0.01), respectively, compared with mice injected with heat-inactivated cytokine.
B6/CBA F1 mice injected with vehicle (sterile saline) before 0.06 or 0.08 nmol bicuculline methiodide (n = six each dose) did not differ in seizure parameters (not shown) from those receiving heat-inactivated cytokines (see Fig. 2). The effects of IL-1Ra and IL-1β on seizures in B6/CBA F1 mice (see Fig. 2) were similar to those observed when these cytokines were injected in wild-type mice littermates of GILRA2 (controls were wild-type littermates receiving heat-inactivated cytokines; n = four each experimental group; not shown).
Seizure Susceptibility in IL-1Ra Overexpressing Mice.
To evaluate whether endogenously produced IL-1Ra has a role in seizure expression, we studied transgenic B6/CBA mice selectively overexpressing the human secretable form of IL-1Ra in astrocytes (GILRA2 strain) under the control of the glial fibrillary acidic protein promoter (16).
Unilateral intrahippocampal injection of bicuculline methiodide induced tonic seizures in 100% of wild-type mice, whereas it was effective in 57% of GILRA2 mice only (P < 0.05 by Fisher's test) (Table 1). The number of tonic seizures in the transgenic animals in the 90-min observation period was decreased by 40% (wild-type, 5 ± 0.5; GILRA2, 3 ± 1.5*, P < 0.05 by Student's t test) and their duration by 76% (P < 0.05; Table 1). The time spent in clonic seizures was reduced by 3-fold (P < 0.01). A significant delay was found in the time to onset of tonic seizures (P < 0.01; Table 1).
Simultaneous EEG recording of hippocampal epileptic activity (14) in randomly chosen mice (Fig. 3) showed that the duration of ictal episodes was significantly reduced in GILRA2 mice (2.8 ± 1.6 min, n = 4, P < 0.05) compared with wild-type mice (8.4 ± 1.5 min, n = 5). Interictal spiking was observed in both experimental groups to a similar extent (spiking activity, min, wild type, 28.0 ± 6.8 vs. GILRA2, 29.3 ± 9.0; frequency, Hz, wild type, 35.0 ± 4.7 vs. GILRA2, 44.0 ± 4.0). The time to onset of ictal activity was delayed by 3.8-fold on average (wild type, min, 2.0 ± 0.8; GILRA2, 7.7 ± 4.4), although this difference was not statistically significant.
The immediate early gene c-fos is a well-established marker of neuronal activation (18). To identify the brain regions affected by seizures, we mapped c-fos expression by in situ hybridization analysis of mRNA after bicuculline injection in wild-type and GILRA2 mice. In wild-type mice, seizures induced a widespread expression of c-fos mRNA, notably in the various hippocampal subfields and in all cortical areas (Fig. 3A). No specific hybridization signal was observed in four of seven GILRA2 mice (Fig. 3B), and a marked reduction was found in the remaining three GILRA2 mice (Fig. 3C).
Quantification of the autoradiograms revealed a reduction in the specific hybridization signal in the hippocampus and cortical areas that was statistically significant only in the frontoparietal cortex (wild-type, arbitrary unit in both hemispheres, 117.0 ± 22.5, n = 5; GILRA2, 15.5 ± 8.8, n = 7, P < 0.01 by Student's t test).
Effect of IL-1Ra on Seizures in IL-1R Type I-Deficient Mice.
Finally, to identify the receptor involved in the anticonvulsant action of IL-1Ra, we assessed its effect on bicuculline-induced seizures in IL-1R type I-deficient mice (17). Table 2 shows that the onset time to motor seizures was delayed by 28% on average (P < 0.05 and P < 0.01) in IL-1RI knockout mice compared with 129/SV PasIco wild-type littermate mice. The other parameters of motor seizures did not significantly differ.
In wild-type 129/SV mice, recombinant IL-1Ra (0.3 nmol) significantly delayed by 2-fold the latency to motor convulsions (P < 0.01) and reduced by 3.9-fold the time spent in tonic seizures (P < 0.01). Differently from B6/CBA mice (Table 1), IL-1Ra did not affect the duration of clonus, suggesting that this component of motor seizures is less susceptible to the pharmacological action of the antagonist.
IL-1Ra also reduced EEG ictal activity [wild-type (W-T), min, 6.4 ± 1.0, n = 15; W-T + IL-1Ra, 3.2 ± 0.7, n = 15; P < 0.01) without modifying interictal spiking (W-T, min, 68.8 ± 9.8; W-T + IL-1Ra, 48.9 ± 7.5). The onset time of ictal activity was significantly delayed (W-T, min, 2.7 ± 0.6; W-T + IL-1Ra, 6.9 ± 1.7, P < 0.05 by Student's t test).
Recombinant IL-1Ra did not affect the motor (Table 2) and EEG (not shown) seizure pattern in IL-1R type I knockout mice.
Discussion
This study shows a powerful anticonvulsant action of IL-1Ra, the naturally occurring antagonist of IL-1β (20), on seizures induced by bicuculline methiodide in mice. IL-1Ra was effective on seizures in various mouse strains, indicating that it acts independently on a specific genetic background.
Both intracerebral infusion of IL-1Ra and its overexpression in astrocytes inhibited motor seizures and ictal activity in the hippocampus without significant changes in interictal spiking. This suggests that, although hippocampal hyperexcitability still occurs, the transition between interictal and ictal activity and the generalization of seizures are specifically impaired by endogenous IL-1Ra. Accordingly, c-fos expression was reduced in the forebrain of GILRA2 mice, particularly in their frontoparietal cortex. Classical antiepileptic drugs such as phenytoin and carbamazepine provide seizure control without modifying interictal epileptiform activity in limbic structures (21).
Besides seizures induced by GABAA receptor antagonism, intracerebral injection of IL-1Ra reduced EEG ictal episodes provoked by intrahippocampal kainate (unpublished data) and motor limbic seizures occurring during status epilepticus in rats (15).
The lack of protective effects of recombinant IL-1Ra in the IL-1R type I-deficient mice clearly indicates that IL-1Ra acts by blocking these receptors in wild-type mice. The distribution of IL-1R type I in the hippocampus on soma and dendrites of granule neurons in the molecular and granular layer of the dentate gyrus (22) places them anatomically in a site crucial for epileptogenesis. Thus, granule neurons play a pivotal role in gating the excitatory drive through the hippocampus, which leads to seizure generalization (23).
The IL-1R type I/IL-1 accessory protein complex binds IL-β with higher affinity than it binds IL-1Ra; in addition, only a few IL-1β-occupied receptor complexes are needed for cellular signaling. Thus, high molar excess (100- to 1,000-fold) of IL-1Ra is needed to counteract IL-1β effects (1, 2). Our experiments are in line with these findings.
It is likely that IL-1β and IL-1Ra are released in the extracellular space after their seizure-induced synthesis, thus enhanced release of other inflammatory cytokines from hippocampal slices of epileptic rats has been described (24).
Several studies of the functional role of IL-1Ra in the brain have shown that it acts by limiting IL-1β-mediated actions, including reduction of ischemic brain damage, inhibition of traumatic brain injury, and excitotoxin-induced neuronal damage (25–29).
Because IL-1β worsens seizures by enhancing glutamate-mediated neurotransmission (14), the anticonvulsant action of IL-1Ra is likely because of antagonism of this effect. The delayed onset of generalized motor seizures in mice lacking IL-1R type I supports the view that the proconvulsant action of endogenous IL-1β is mediated by these receptors.
IL-1Ra is rapidly and reversibly induced in the hippocampus after seizures (12, 13, 15). Differently from septic shock or inflammatory diseases where the circulating IL-1Ra levels are 100-fold in excess of those of IL-1β (2), seizures increase IL-1Ra in the hippocampus to a similar (15) or even lower (30) extent than IL-1β. Thus, the brain appears to be less effective in inducing IL-1Ra as a mechanism to terminate IL-1β actions than the periphery.
We suggest that the balance between IL-1β and IL-1Ra during seizures plays a significant role in altering neuronal network excitability, thus affecting the maintenance and spread of seizures.
Although the expression of inflammatory cytokines has not yet been investigated in human epileptic brain tissue, an increased production of IL-1α and a functional activation of microglia and astrocytes (the main sources of cytokines in the brain) have been reported in human temporal lobe epilepsy (31, 32). The hippocampus is crucially involved in this common form of epilepsy, which is highly resistant to classical anticonvulsant treatment (21).
Changes in the IL-1Ra/IL-1β ratio may therefore represent an effective physiopathological mechanism to control seizures, and this may be of pharmacological relevance. Because IL-1Ra seems to be a well-tolerated endogenous protein that is highly inducible (2), we suggest that pharmacological means that increase the IL-1Ra/IL-1β ratio may be useful in inhibiting seizures.
Acknowledgments
The authors are grateful for the skillful technical contribution of Mr. A. Borroni to part of these experiments and the dedicated and invaluable assistance of Mr. G. Gambaro for the EEG setup. We thank Drs. S. Garattini, R. Samanin (Mario Negri Institute for Pharmacological Research, Milano) and M. de Curtis (Istituto Neurologico “Carlo Besta,” Milano) for critically reviewing this manuscript. This study was supported by the Telethon Onlus Foundation (Grant no. E.1094) and the Swedish Medical Research Council (Grant no. 13122).
Abbreviations
- IL-1Ra
IL-1 receptor antagonist
- EEG
electroencephalographic
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190206797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190206797
References
- 1.Rothwell N J, Hopkins S J. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 3.Scarborough D E, Lee S L, Dinarello C A, Reichlin S. Endocrinology. 1989;124:549–551. doi: 10.1210/endo-124-1-549. [DOI] [PubMed] [Google Scholar]
- 4.Spranger M, Lindholm D, Bandtlow C, Heumann R, Gnahn H, Naher-Noè M, Thoenen H. Eur J Neurosci. 1990;2:69–76. doi: 10.1111/j.1460-9568.1990.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 5.Lapchak P A, Araujo D M, Hefti F. Neuroscience. 1993;53:297–301. doi: 10.1016/0306-4522(93)90196-m. [DOI] [PubMed] [Google Scholar]
- 6.Coogan A, O'Connor J J. NeuroReport. 1997;8:2107–2110. doi: 10.1097/00001756-199707070-00004. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Cheng Q, Malik S, Yang J. J Pharmacol Exp Ther. 2000;292:497–504. [PubMed] [Google Scholar]
- 8.Zeise M L, Espinoza J, Morales P, Nalli A. Brain Res. 1997;768:341–344. doi: 10.1016/s0006-8993(97)00787-7. [DOI] [PubMed] [Google Scholar]
- 9.Miller L G, Galpern W R, Dunlap K, Dinarello C A, Turner T J. Mol Pharmacol. 1991;39:105–108. [PubMed] [Google Scholar]
- 10.Minami M, Kuraishi Y, Yamaguchi T, Nakai S, Hirai Y, Sato M. Biochem Biophys Res Comm. 1990;171:823–827. doi: 10.1016/0006-291x(90)91221-d. [DOI] [PubMed] [Google Scholar]
- 11.Nishiyori A, Minami M, Takami S, Satoh M. Mol Brain Res. 1997;58:237–245. doi: 10.1016/s0169-328x(97)00195-2. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson C, Winblad B, Schultzberg M. Mol Brain Res. 1998;58:195–208. doi: 10.1016/s0169-328x(98)00125-9. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson C, Van Dam A M, Lucassen P J, Bol J G, Winblad B, Schultzberg M. Neuroscience. 1999;93:915–930. doi: 10.1016/s0306-4522(99)00178-5. [DOI] [PubMed] [Google Scholar]
- 14.Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni M G. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Simoni M G, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, De Luigi A, Garattini S, Vezzani A. Eur J Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- 16.Lundkvist J, Sundgren-Andersson A K, Tingsborg S, Ostlund P, Engfors C, Alheim K, Bartfai T, Iverfeldt K, Schultzberg M. Am J Physiol. 1999;276:R644–R651. doi: 10.1152/ajpregu.1999.276.3.R644. [DOI] [PubMed] [Google Scholar]
- 17.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan E B, Bartfai T, Solorzano C, Moldawer L L, Chizzonite R, et al. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 18.Morgan J I, Cohen D R, Hempstead J L, Curran T. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 19.Curtis D R, Duggan A W, Felix D, Johnston G A. Nature (London) 1970;226:1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg S P, Evans R J, Arend W P, Verber E, Brewer M T, Hannun C H, Thompson R C. Nature (London) 1990;343:341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- 21.Bazil C W, Pedley T A. In: Antiepileptic Drugs. Levy R H, Mattson R H, Meldrum B S, editors. New York: Raven; 1995. pp. 79–89. [Google Scholar]
- 22.Ban E, Milon G, Prudhomme N, Fillion G, Haour F. Neuroscience. 1991;43:21–30. doi: 10.1016/0306-4522(91)90412-h. [DOI] [PubMed] [Google Scholar]
- 23.McNamara J O. J Neurosci. 1994;14:3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bock F, Dornand J, Rondouin G. NeuroReport. 1996;7:1125–1129. doi: 10.1097/00001756-199604260-00004. [DOI] [PubMed] [Google Scholar]
- 25.Loddick S A, Rothwell N J. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Martin D, Chinookoswong N, Miller G. Exp Neurol. 1994;130:362–367. doi: 10.1006/exnr.1994.1215. [DOI] [PubMed] [Google Scholar]
- 27.Toulmond S, Rothwell N J. Brain Res. 1995;671:261–266. doi: 10.1016/0006-8993(94)01343-g. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence C B, Allan S M, Rothwell N J. Eur J Neurosci. 1998;10:1188–1195. doi: 10.1046/j.1460-9568.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- 29.Panegyres P K, Hughes J. J Neurol Sci. 1998;154:123–132. doi: 10.1016/s0022-510x(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 30.Plata-Salaman C R, Ilyin S E, Turrin N P, Gayle D, Flynn M C, Romanovitch A E, Kelly M E, Bureau Y, Anisman H, McIntyre D. Mol Brain Res. 2000;75:248–258. doi: 10.1016/s0169-328x(99)00306-x. [DOI] [PubMed] [Google Scholar]
- 31.Sheng J G, Boop F A, Mrak R E, Griffin W S. J Neurochem. 1994;63:1872–1879. doi: 10.1046/j.1471-4159.1994.63051872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brotchi J. Acta Neurol Belg. 1979;79:137–304. [PubMed] [Google Scholar]