Abstract
AIMS—To examine a large family with an autosomal dominant fundus dystrophy and to investigate whether or not mutations in TIMP-3 gene were involved. METHODS—A large family of 58 individuals with an autosomal dominant fundus dystrophy was examined ophthalmologically. A DNA linkage analysis in the 22q12.1-q13.2 region was performed. The TIMP-3 gene was screened for mutations in all five exons. RESULTS—In this large family 15 individuals were affected. All other individuals were found to be clinically unaffected. Pisciform flecks in the midperiphery and drusen-like deposits were the most typical ophthalmological finding in this family and were encountered from the fifth decade on. Chorioretinal atrophy and neovascularisation with disciform lesions characterised the disease from the sixth decade on. Linkage analysis using an affected only analysis, showed a maximum positive lod score of 3.94 at θ = 0.0 with marker D22S283. No mutations possibly causing Sorsby fundus dystrophy were found in either the exonic sequences, the promotor region, or the 3'UTR. CONCLUSION—The family in this pedigree has an autosomal dominant fundus dystrophy, which is most probably Sorsby fundus dystrophy. Although, in the linkage analysis, significant positive lod scores were found with the region 22q12.1-q13.2, no causative mutations could be identified in the TIMP-3 gene.
Full Text
The Full Text of this article is available as a PDF (221.3 KB).
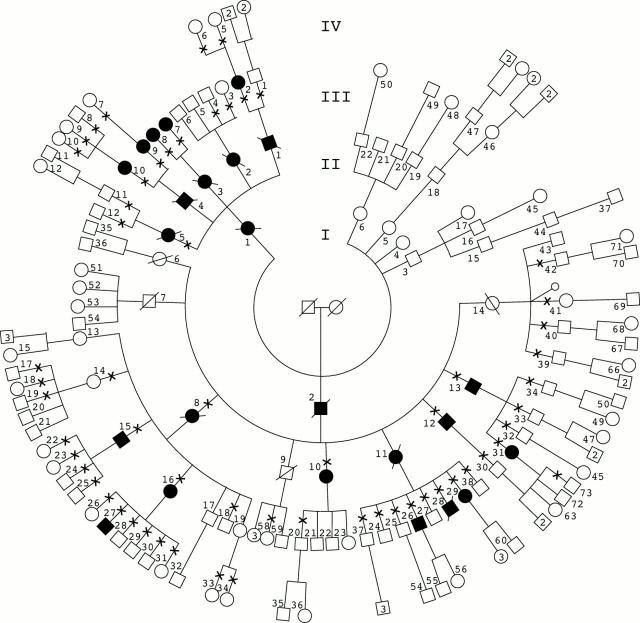
Figure 1 .
Pedigree of the family with Sorsby fundus dystrophy. Males are represented by squares and females are represented by circles; solid symbols represent individuals with Sorsby fundus dystrophy, open symbols represent normal individuals. A cross represents the individuals who were clinically examined and used for linkage analysis.
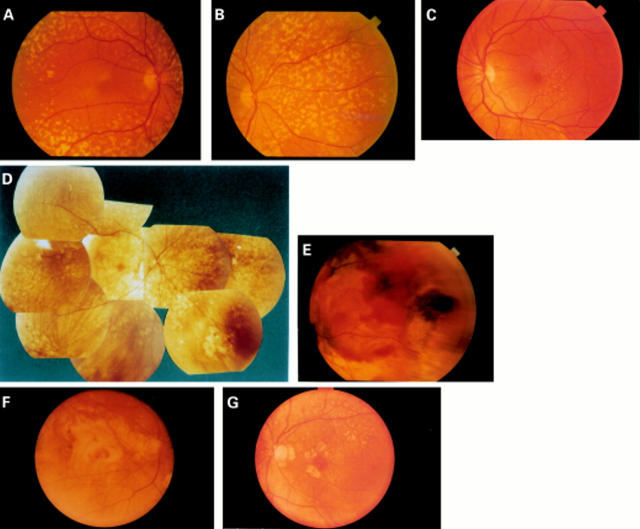
Figure 2 .
(A) Pisciform flecks at the posterior pole (patient III,29). (B) Flecks nasally to the pupil (patient III,29). (C) Drusen-like deposits (patient III,31). (D) Extensive chorioretinal atrophy (patient III,9). (E) Choroidal neovascularisation with haemorrhages and exudation (patient III,7). (F) Disciform lesion with fibrotic reaction extending far outside the macula (patient II,8 right eye). (G) Pisciform flecks at the arcades, atrophic patches surrounding the macula (patient II,8 left eye).
Figure 3 .
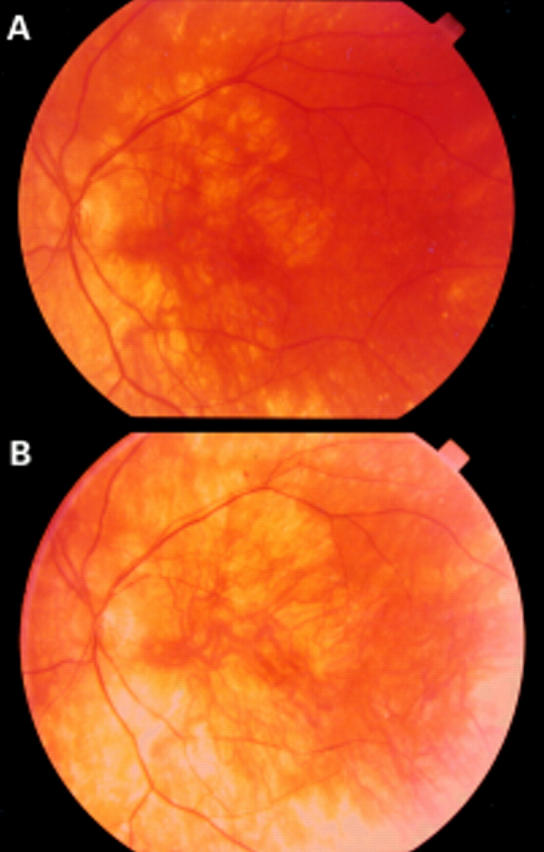
(A) Patient III,9 at the age of 60 years; left eye visual acuity 0.2, notice atrophy of the capillary. (B) Same patient (III,9) at the age of 63; left eye visual acuity decreased to light perception due to involvement of the macula by the choriocapillary atrophy.
Figure 4 .
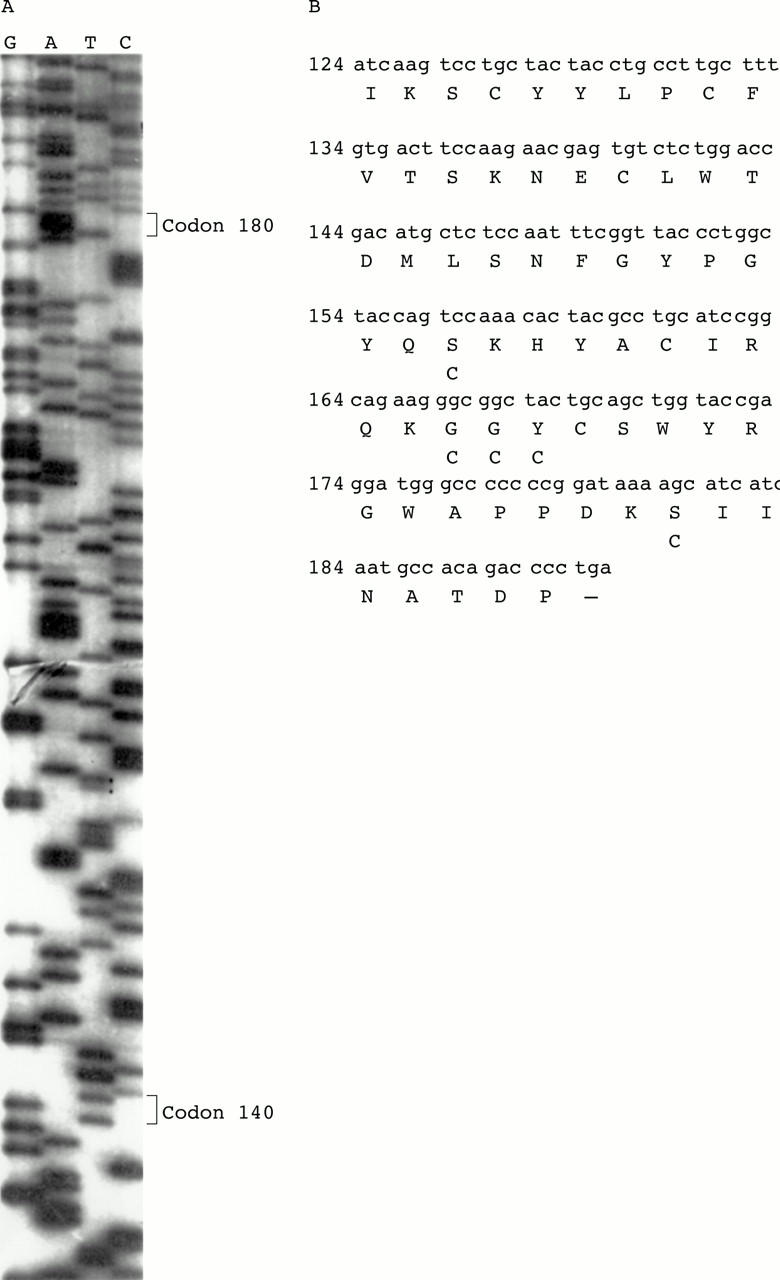
(A) Sequence of exon 5 in an affected individual with Sorsby fundus dystrophy. (B) Nucleotide sequence and sequence of the conceptual translation product of exon 5. For previously described mutations, the replacing amino acid is shown underneath the consensus amino acid.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte S. S., Mattei M. G., Olsen B. R. Cloning of the cDNA encoding human tissue inhibitor of metalloproteinases-3 (TIMP-3) and mapping of the TIMP3 gene to chromosome 22. Genomics. 1994 Jan 1;19(1):86–90. doi: 10.1006/geno.1994.1016. [DOI] [PubMed] [Google Scholar]
- Bergen A. A., Van den Born L. I., Schuurman E. J., Pinckers A. J., Van Ommen G. J., Bleekers-Wagemakers E. M., Sandkuijl L. A. Multipoint linkage analysis and homogeneity tests in 15 Dutch X-linked retinitis pigmentosa families. Ophthalmic Genet. 1995 Jun;16(2):63–70. doi: 10.3109/13816819509056914. [DOI] [PubMed] [Google Scholar]
- Capon M. R., Marshall J., Krafft J. I., Alexander R. A., Hiscott P. S., Bird A. C. Sorsby's fundus dystrophy. A light and electron microscopic study. Ophthalmology. 1989 Dec;96(12):1769–1777. doi: 10.1016/s0161-6420(89)32664-9. [DOI] [PubMed] [Google Scholar]
- Capon M. R., Polkinghorne P. J., Fitzke F. W., Bird A. C. Sorsby's pseudoinflammatory macula dystrophy--Sorsby's fundus dystrophies. Eye (Lond) 1988;2(Pt 1):114–122. doi: 10.1038/eye.1988.23. [DOI] [PubMed] [Google Scholar]
- Carrero-Valenzuela R. D., Klein M. L., Weleber R. G., Murphey W. H., Litt M. Sorsby fundus dystrophy. A family with the Ser181Cys mutation of the tissue inhibitor of metalloproteinases 3. Arch Ophthalmol. 1996 Jun;114(6):737–738. doi: 10.1001/archopht.1996.01100130729016. [DOI] [PubMed] [Google Scholar]
- De La Paz M. A., Pericak-Vance M. A., Lennon F., Haines J. L., Seddon J. M. Exclusion of TIMP3 as a candidate locus in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997 May;38(6):1060–1065. [PubMed] [Google Scholar]
- Felbor U., Stöhr H., Amann T., Schönherr U., Apfelstedt-Sylla E., Weber B. H. A second independent Tyr168Cys mutation in the tissue inhibitor of metalloproteinases-3 (TIMP3) in Sorsby's fundus dystrophy. J Med Genet. 1996 Mar;33(3):233–236. doi: 10.1136/jmg.33.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felbor U., Stöhr H., Amann T., Schönherr U., Weber B. H. A novel Ser156Cys mutation in the tissue inhibitor of metalloproteinases-3 (TIMP3) in Sorsby's fundus dystrophy with unusual clinical features. Hum Mol Genet. 1995 Dec;4(12):2415–2416. doi: 10.1093/hmg/4.12.2415. [DOI] [PubMed] [Google Scholar]
- Felbor U., Suvanto E. A., Forsius H. R., Eriksson A. W., Weber B. H. Autosomal recessive Sorsby fundus dystrophy revisited: molecular evidence for dominant inheritance. Am J Hum Genet. 1997 Jan;60(1):57–62. [PMC free article] [PubMed] [Google Scholar]
- Fraser H. B., Wallace D. C. Sorsby's familial pseudo-inflammatory macular dystrophy. Am J Ophthalmol. 1971 Jun;71(6):1216–1220. doi: 10.1016/0002-9394(71)90965-2. [DOI] [PubMed] [Google Scholar]
- Gregory C. Y., Wijesuriya S., Evans K., Jay M., Bird A. C., Bhattacharya S. S. Linkage refinement localises Sorsby fundus dystrophy between markers D22S275 and D22S278. J Med Genet. 1995 Mar;32(3):240–241. doi: 10.1136/jmg.32.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. K., Ewing C. C., Ives E. J., Carruthers J. D. Sorsby's fundus dystrophy. Ophthalmology. 1989 Dec;96(12):1755–1762. doi: 10.1016/s0161-6420(89)32647-9. [DOI] [PubMed] [Google Scholar]
- Hoskin A., Sehmi K., Bird A. C. Sorsby's pseudoinflammatory macular dystrophy. Br J Ophthalmol. 1981 Dec;65(12):859–865. doi: 10.1136/bjo.65.12.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S. G., Cideciyan A. V., Regunath G., Rodriguez F. J., Vandenburgh K., Sheffield V. C., Stone E. M. Night blindness in Sorsby's fundus dystrophy reversed by vitamin A. Nat Genet. 1995 Sep;11(1):27–32. doi: 10.1038/ng0995-27. [DOI] [PubMed] [Google Scholar]
- Kalmus H., Seedburgh D. Probable common origin of a hereditary fundus dystrophy (Sorsby's familial pseudoinflammatory macular dystrophy) in an English and Australian family. J Med Genet. 1976 Aug;13(4):271–276. doi: 10.1136/jmg.13.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Klein B. E., Linton K. L. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992 Jun;99(6):933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- Polkinghorne P. J., Capon M. R., Berninger T., Lyness A. L., Sehmi K., Bird A. C. Sorsby's fundus dystrophy. A clinical study. Ophthalmology. 1989 Dec;96(12):1763–1768. doi: 10.1016/s0161-6420(89)32654-6. [DOI] [PubMed] [Google Scholar]
- SORSBY A., MASON M. E. J. A fundus dystrophy with unusual features. Br J Ophthalmol. 1949 Feb;33(2):67–97. doi: 10.1136/bjo.33.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr H., Roomp K., Felbor U., Weber B. H. Genomic organization of the human tissue inhibitor of metalloproteinases-3 (TIMP3) Genome Res. 1995 Dec;5(5):483–487. doi: 10.1101/gr.5.5.483. [DOI] [PubMed] [Google Scholar]
- Tabata Y., Isashiki Y., Kamimura K., Nakao K., Ohba N. A novel splice site mutation in the tissue inhibitor of the metalloproteinases-3 gene in Sorsby's fundus dystrophy with unusual clinical features. Hum Genet. 1998 Aug;103(2):179–182. doi: 10.1007/pl00008707. [DOI] [PubMed] [Google Scholar]
- Vingerling J. R., Dielemans I., Hofman A., Grobbee D. E., Hijmering M., Kramer C. F., de Jong P. T. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995 Feb;102(2):205–210. doi: 10.1016/s0161-6420(95)31034-2. [DOI] [PubMed] [Google Scholar]
- Weber B. H., Vogt G., Pruett R. C., Stöhr H., Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet. 1994 Dec;8(4):352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- Weber B. H., Vogt G., Wolz W., Ives E. J., Ewing C. C. Sorsby's fundus dystrophy is genetically linked to chromosome 22q13-qter. Nat Genet. 1994 Jun;7(2):158–161. doi: 10.1038/ng0694-158. [DOI] [PubMed] [Google Scholar]
- van Soest S., van den Born L. I., Gal A., Farrar G. J., Bleeker-Wagemakers L. M., Westerveld A., Humphries P., Sandkuijl L. A., Bergen A. A. Assignment of a gene for autosomal recessive retinitis pigmentosa (RP12) to chromosome 1q31-q32.1 in an inbred and genetically heterogeneous disease population. Genomics. 1994 Aug;22(3):499–504. doi: 10.1006/geno.1994.1422. [DOI] [PubMed] [Google Scholar]