Abstract
BACKGROUND—The histological characteristics of ocular adnexal lymphomas have previously provided only a limited guide to clinical outcome for affected patients. This clinicopathological relation was re-examined using the Revised European American Lymphoma (REAL) system to classify the tumours in a large cohort of patients. METHODS—The biopsies and clinical follow up data for 192 patients with ocular adnexal lymphoma were reviewed, the biopsies being regraded in accordance with the REAL classification. For each of five histological groups, logistic regression analysis was used to determine the odds ratios (OR) for the presence of systemic disease at the time of orbital diagnosis and Cox regression analysis was used to assess the hazard ratios (HR) for disseminated disease and lymphoma related death. For 108 patients in whom extraorbital spread occurred, the histological category of lymphoma was compared with the sites of dissemination. RESULTS—At presentation, the frequency of previous or concurrent extraorbital disease increased from marginal zone lymphoma (OR 1.0), diffuse lymphoplasmacytic/lymphoplasmacytoid lymphoma (OR 2.3), follicle centre lymphoma (OR 3.8), diffuse large B cell lymphoma (OR 4.0) to other histological lymphoma variants (OR 26.8). For all histological types, the estimated risk of extraorbital disease and lymphoma related death continued for many years and the proportion of patients with at least one extraorbital recurrence after 5 years was 47% for MZL, 48% for LPL, 64% for FCL, 81% for DLCL, and 95% for other lymphoma variants. The corresponding estimated rates for 5 year lymphoma related mortality were 12%, 19%, 22%, 48%, and 53% respectively. CONCLUSIONS—Patients with ocular adnexal lymphoma can be classified by REAL into five distinct groups, which show a progressive increase in the risks of extraorbital disease at diagnosis, of disease dissemination with time, and of tumour related death.
Full Text
The Full Text of this article is available as a PDF (235.5 KB).
Figure 1 .
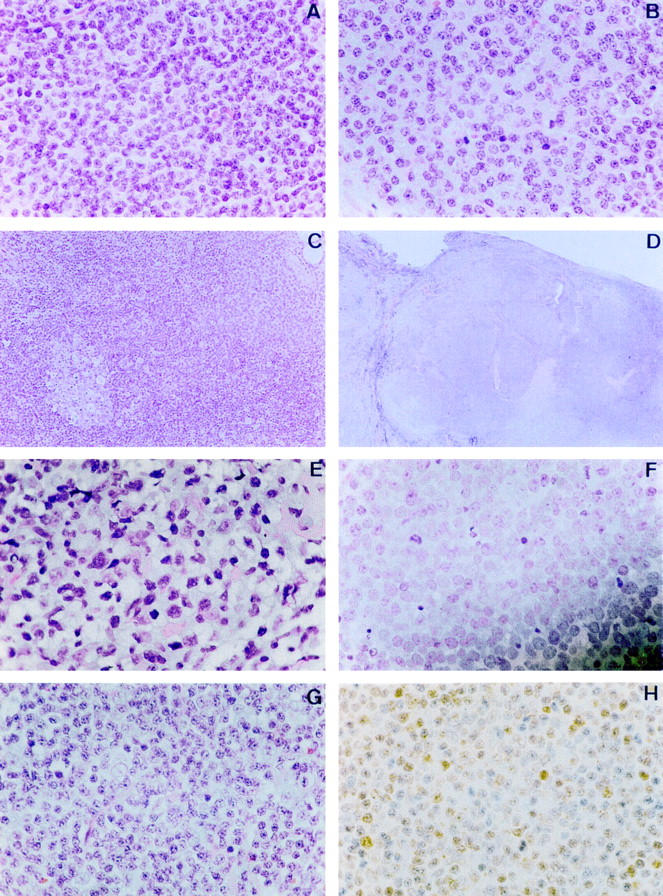
Typical histomorphology for lymphomas of the ocular adnexa (haematoxylin and eosin staining, unless otherwise stated). (A) Centrocyte-like cells in marginal zone lymphoma; (B) lymphoplasmacytoid cells in marginal zone lymphoma; (C) trapped reactive follicle in marginal zone lymphoma; (D) follicular architecture in follicle centre lymphoma; (E) centrocytes and centroblasts in follicle centre lymphoma; (F) centroblasts in diffuse large B cell lymphoma; (G) centrocytes in mantle cell lymphoma; (H) Cyclin D-1 staining in mantle cell lymphoma.
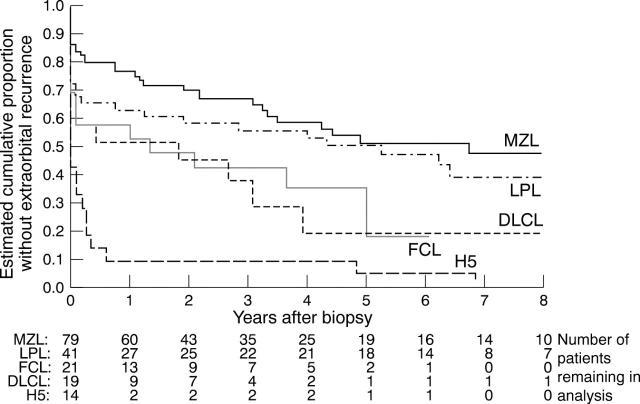
Figure 2 .
Cumulative proportion without extraorbital disease, based on 192 patients with ocular adnexal lymphoma: 82 marginal zone lymphomas (MZL), 44 lymphoplasmacytoid/lymphoplasmacytic lymphomas (LPL), 26 follicle centre lymphomas (FCL), 19 diffuse large B cell lymphomas (DLCL), and 21 other variants (H5). The data are censored for patients who, at time of last follow up, had not developed extraorbital disease. The figures refer, at a given time, to the number of survivors without recurrent extraorbital disease (classified by histopathological type).
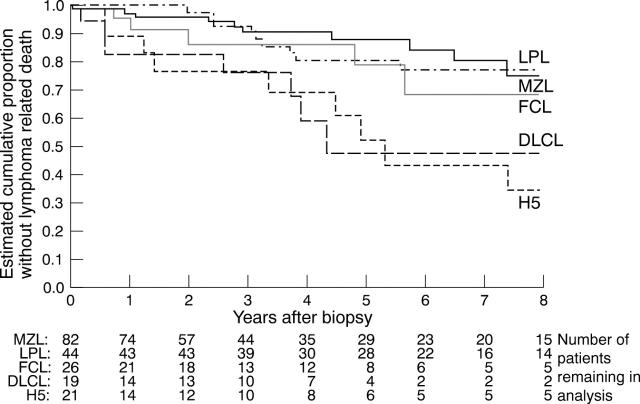
Figure 3 .
Cumulative proportion without lymphoma related death, based on 192 patients with ocular adnexal lymphoma (abbreviations and numbers as Fig 2). The data were censored for patients in whom the last follow up was before the end of the 8 year study period, either because of incomplete follow up or where death was unrelated to lymphoma. The numerical data refer to the number of patients alive at a given time.
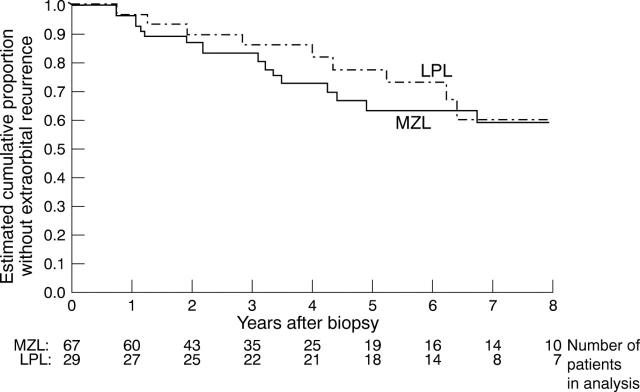
Figure 4 .
Cumulative proportion of patients, presenting with solely orbital disease, who remain without extraorbital spread—a comparison of marginal zone lymphoma (MZL; 67 cases) and lymphoplasmacytoid/lymphoplasmacytic lymphoma (LPL; 29 cases). The figures refer to the number of patients alive without recurrence at a given time.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Advani S. H., Iyer R. S., Gopal R., Nair C. N., Saikia T., Dinshaw K. A., Kurkure P. A., Pai S. K. Multifocal extranodal lymphomas: an expression of homing phenomenon. Oncology. 1990;47(4):334–338. doi: 10.1159/000226844. [DOI] [PubMed] [Google Scholar]
- Berger F., Felman P., Sonet A., Salles G., Bastion Y., Bryon P. A., Coiffier B. Nonfollicular small B-cell lymphomas: a heterogeneous group of patients with distinct clinical features and outcome. Blood. 1994 May 15;83(10):2829–2835. [PubMed] [Google Scholar]
- Bessell E. M., Henk J. M., Wright J. E., Whitelocke R. A. Orbital and conjunctival lymphoma treatment and prognosis. Radiother Oncol. 1988 Dec;13(4):237–244. doi: 10.1016/0167-8140(88)90218-6. [DOI] [PubMed] [Google Scholar]
- Chan J. K., Ng C. S., Isaacson P. G. Relationship between high-grade lymphoma and low-grade B-cell mucosa-associated lymphoid tissue lymphoma (MALToma) of the stomach. Am J Pathol. 1990 May;136(5):1153–1164. [PMC free article] [PubMed] [Google Scholar]
- Chen P. M., Liu J. H., Lin S. H., Hsu W. M., Kao S. C. Rearrangements of immunoglobulin gene and oncogenes in ocular adnexal pseudolymphoma. Curr Eye Res. 1991 Jun;10(6):547–555. doi: 10.3109/02713689109001763. [DOI] [PubMed] [Google Scholar]
- Cogliatti S. B., Schmid U., Schumacher U., Eckert F., Hansmann M. L., Hedderich J., Takahashi H., Lennert K. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology. 1991 Nov;101(5):1159–1170. doi: 10.1016/0016-5085(91)90063-q. [DOI] [PubMed] [Google Scholar]
- Cohen P. L., Brooks J. J. Lymphomas of the breast. A clinicopathologic and immunohistochemical study of primary and secondary cases. Cancer. 1991 Mar 1;67(5):1359–1369. doi: 10.1002/1097-0142(19910301)67:5<1359::aid-cncr2820670515>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Coupland S. E., Krause L., Delecluse H. J., Anagnostopoulos I., Foss H. D., Hummel M., Bornfeld N., Lee W. R., Stein H. Lymphoproliferative lesions of the ocular adnexa. Analysis of 112 cases. Ophthalmology. 1998 Aug;105(8):1430–1441. doi: 10.1016/S0161-6420(98)98024-1. [DOI] [PubMed] [Google Scholar]
- Du M., Peng H., Singh N., Isaacson P. G., Pan L. The accumulation of p53 abnormalities is associated with progression of mucosa-associated lymphoid tissue lymphoma. Blood. 1995 Dec 15;86(12):4587–4593. [PubMed] [Google Scholar]
- Evans H. L. Extranodal small lymphocytic proliferation: a clinicopathologic and immunocytochemical study. Cancer. 1982 Jan 1;49(1):84–96. doi: 10.1002/1097-0142(19820101)49:1<84::aid-cncr2820490119>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Fellbaum C., Hansmann M. L., Lennert K. Malignant lymphomas of the nasal cavity and paranasal sinuses. Virchows Arch A Pathol Anat Histopathol. 1989;414(5):399–405. doi: 10.1007/BF00718623. [DOI] [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Hyjek E., Smith W. J., Isaacson P. G. Primary B-cell lymphoma of salivary glands and its relationship to myoepithelial sialadenitis. Hum Pathol. 1988 Jul;19(7):766–776. doi: 10.1016/s0046-8177(88)80259-4. [DOI] [PubMed] [Google Scholar]
- Isaacson P. G. Lymphomas of mucosa-associated lymphoid tissue (MALT). Histopathology. 1990 Jun;16(6):617–619. doi: 10.1111/j.1365-2559.1990.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Isaacson P. G., Spencer J. Malignant lymphoma of mucosa-associated lymphoid tissue. Histopathology. 1987 May;11(5):445–462. doi: 10.1111/j.1365-2559.1987.tb02654.x. [DOI] [PubMed] [Google Scholar]
- Isaacson P. G., Wotherspoon A. C., Diss T. C., Pan L. X. Bcl-2 expression in lymphomas. Lancet. 1991 Jan 19;337(8734):175–176. doi: 10.1016/0140-6736(91)90838-g. [DOI] [PubMed] [Google Scholar]
- Isaacson P. G., Wotherspoon A. C., Diss T., Pan L. X. Follicular colonization in B-cell lymphoma of mucosa-associated lymphoid tissue. Am J Surg Pathol. 1991 Sep;15(9):819–828. doi: 10.1097/00000478-199109000-00001. [DOI] [PubMed] [Google Scholar]
- Isaacson P., Wright D. H. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983 Oct 15;52(8):1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Jakobiec F. A., Iwamoto T., Patell M., Knowles D. M., 2nd Ocular adnexal monoclonal lymphoid tumors with a favorable prognosis. Ophthalmology. 1986 Dec;93(12):1547–1557. doi: 10.1016/s0161-6420(86)33532-2. [DOI] [PubMed] [Google Scholar]
- Knowles D. M., 2nd The extranodal lymphoid infiltrate: a diagnostic dilemma. Semin Diagn Pathol. 1985 Aug;2(3):147–151. [PubMed] [Google Scholar]
- Knowles D. M., Athan E., Ubriaco A., McNally L., Inghirami G., Wieczorek R., Finfer M., Jakobiec F. A. Extranodal noncutaneous lymphoid hyperplasias represent a continuous spectrum of B-cell neoplasia: demonstration by molecular genetic analysis. Blood. 1989 May 1;73(6):1635–1645. [PubMed] [Google Scholar]
- Knowles D. M., Jakobiec F. A., McNally L., Burke J. S. Lymphoid hyperplasia and malignant lymphoma occurring in the ocular adnexa (orbit, conjunctiva, and eyelids): a prospective multiparametric analysis of 108 cases during 1977 to 1987. Hum Pathol. 1990 Sep;21(9):959–973. doi: 10.1016/0046-8177(90)90181-4. [DOI] [PubMed] [Google Scholar]
- SALTZSTEIN S. L. PULMONARY MALIGNANT LYMPHOMAS AND PSEUDOLYMPHOMAS: CLASSIFICATION, THERAPY, AND PROGNOSIS. Cancer. 1963 Jul;16:928–955. doi: 10.1002/1097-0142(196307)16:7<928::aid-cncr2820160709>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Schmid C., Kirkham N., Diss T., Isaacson P. G. Splenic marginal zone cell lymphoma. Am J Surg Pathol. 1992 May;16(5):455–466. doi: 10.1097/00000478-199205000-00004. [DOI] [PubMed] [Google Scholar]
- Tolksdorf G., Stein H., Lennert K. Morphological and immunological definition of a malignant lymphoma derived from germinal-centre cells with cleaved nuclei (centrocytes). Br J Cancer. 1980 Feb;41(2):168–182. doi: 10.1038/bjc.1980.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White V. A., Gascoyne R. D., McNeil B. K., Chang W. Y., Brewer L. V., Rootman J. Histopathologic findings and frequency of clonality detected by the polymerase chain reaction in ocular adnexal lymphoproliferative lesions. Mod Pathol. 1996 Nov;9(11):1052–1061. [PubMed] [Google Scholar]
- White W. L., Ferry J. A., Harris N. L., Grove A. S., Jr Ocular adnexal lymphoma. A clinicopathologic study with identification of lymphomas of mucosa-associated lymphoid tissue type. Ophthalmology. 1995 Dec;102(12):1994–2006. doi: 10.1016/s0161-6420(95)30764-6. [DOI] [PubMed] [Google Scholar]
- Yang W. I., Zukerberg L. R., Motokura T., Arnold A., Harris N. L. Cyclin D1 (Bcl-1, PRAD1) protein expression in low-grade B-cell lymphomas and reactive hyperplasia. Am J Pathol. 1994 Jul;145(1):86–96. [PMC free article] [PubMed] [Google Scholar]
- Zukerberg L. R., Medeiros L. J., Ferry J. A., Harris N. L. Diffuse low-grade B-cell lymphomas. Four clinically distinct subtypes defined by a combination of morphologic and immunophenotypic features. Am J Clin Pathol. 1993 Oct;100(4):373–385. doi: 10.1093/ajcp/100.4.373. [DOI] [PubMed] [Google Scholar]