Abstract
BACKGROUND—Several ocular side effects including uveitis, have been reported following topical β blocker treatment for glaucoma and ocular hypertension. The incidence of these side effects was investigated in the Netherlands. METHODS—A prospective observational design was used whereby monthly questionnaires were sent to all practising ophthalmologists in the Netherlands during 3 consecutive months. Questionnaires were returned at the end of each month. Any patient whose topical β blocker therapy was altered because of an ocular reaction was noted on this questionnaire. Ophthalmologists who did not return their questionnaires were interviewed by telephone at the end of the study period. The number of patients using topical β blockers was derived from drug sales figures. RESULTS—70% (328/467) of the ophthalmologists in the Netherlands participated in the study. During the 3 month study period 34 cases were reported: 15 patients had periorbital dermatitis, in eight patients eyelids and conjunctiva were affected, in seven patients the conjunctiva was affected, and four patients had punctate keratitis. The calculated incidence of ocular side effects during topical β blocker therapy was 1.51 cases/1000 patient years. CONCLUSION—Topical β blocker therapy is associated with few clinically important ocular side effects. No cases of uveitis were reported.
Full Text
The Full Text of this article is available as a PDF (129.3 KB).
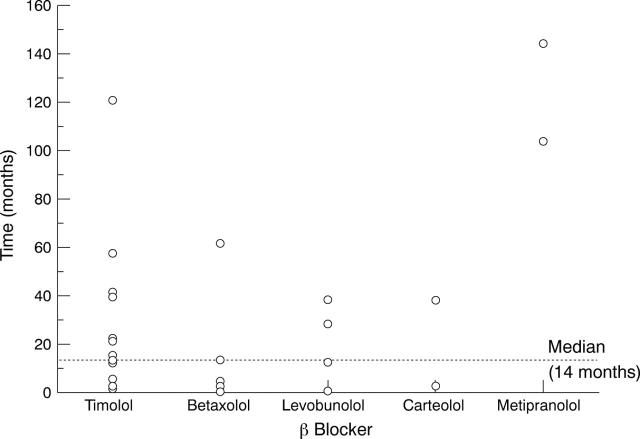
Figure 1 .
Time that patients used the topical β blocker before it was altered because of ocular side effects. For seven patients this information could not be obtained (n=27). Median time the patients used their topical β blocker was 14 months (range 1-144 months).
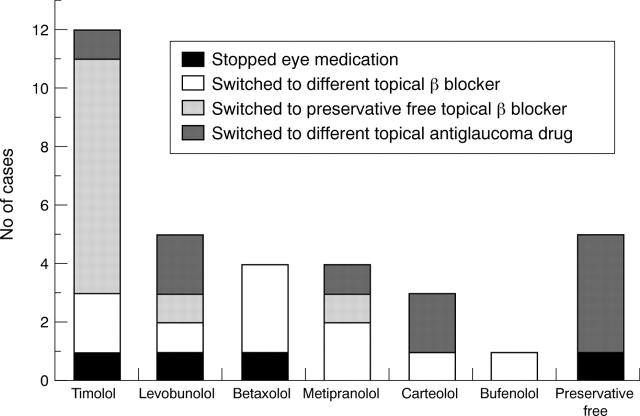
Figure 2 .
Switches made in topical β blocker therapy because of an ocular side effect (n=34). Preservative free denotes all patients altering their preservative-free β blocker medication because of an ocular side effect. In this latter group, four patients used preservative-free timolol and one patient used preservative-free metipranolol. Whether the patient was prescribed a different β blocker, a preservative-free β blocker, a different topical antiglaucoma drug, or stopped topical antiglaucoma therapy is also shown.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akingbehin T., Villada J. R. Metipranolol-associated granulomatous anterior uveitis. Br J Ophthalmol. 1991 Sep;75(9):519–523. doi: 10.1136/bjo.75.9.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R. W., Moke P., Blair R. C., Nissenbaum R. Uveitis associated with topical beta-blockers. Arch Ophthalmol. 1996 Oct;114(10):1181–1182. doi: 10.1001/archopht.1996.01100140381002. [DOI] [PubMed] [Google Scholar]
- Burvenich H. Metipranolol associated granulomatous anterior uveitis: not so uncommon as thought. Bull Soc Belge Ophtalmol. 1995;257:63–67. [PubMed] [Google Scholar]
- Derous D., de Keizer R. J., de Wolff-Rouendaal D., Soudijn W. Conjunctival keratinisation, an abnormal reaction to an ocular beta-blocker. Acta Ophthalmol (Copenh) 1989 Jun;67(3):333–338. doi: 10.1111/j.1755-3768.1989.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Fiore P. M., Jacobs I. H., Goldberg D. B. Drug-induced pemphigoid. A spectrum of diseases. Arch Ophthalmol. 1987 Dec;105(12):1660–1663. doi: 10.1001/archopht.1987.01060120058023. [DOI] [PubMed] [Google Scholar]
- Herbst R. A., Maibach H. I. Contact dermatitis caused by allergy to ophthalmics: an update. Contact Dermatitis. 1992 Nov;27(5):335–336. doi: 10.1111/j.1600-0536.1992.tb03299.x. [DOI] [PubMed] [Google Scholar]
- Jain S. Betaxolol-associated anterior uveitis. Eye (Lond) 1994;8(Pt 6):708–709. doi: 10.1038/eye.1994.181. [DOI] [PubMed] [Google Scholar]
- Krieglstein G. K., Novack G. D., Voepel E., Schwarzbach G., Lange U., Schunck K. P., Lue J. C., Glavinos E. P. Levobunolol and metipranolol: comparative ocular hypotensive efficacy, safety, and comfort. Br J Ophthalmol. 1987 Apr;71(4):250–253. doi: 10.1136/bjo.71.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens E. V., van Best J. A., Sterk C. C., de Keizer R. J. Decreased basal tear turnover in patients with untreated primary open-angle glaucoma. Am J Ophthalmol. 1995 Jul;120(1):41–46. doi: 10.1016/s0002-9394(14)73757-2. [DOI] [PubMed] [Google Scholar]
- Melles R. B., Wong I. G. Metipranolol-associated granulomatous iritis. Am J Ophthalmol. 1994 Dec 15;118(6):712–715. doi: 10.1016/s0002-9394(14)72549-8. [DOI] [PubMed] [Google Scholar]
- Nelson W. L., Fraunfelder F. T., Sills J. M., Arrowsmith J. B., Kuritsky J. N. Adverse respiratory and cardiovascular events attributed to timolol ophthalmic solution, 1978-1985. Am J Ophthalmol. 1986 Nov 15;102(5):606–611. doi: 10.1016/0002-9394(86)90532-5. [DOI] [PubMed] [Google Scholar]
- O'Donnell B. F., Foulds I. S. Contact allergy to beta-blocking agents in ophthalmic preparations. Contact Dermatitis. 1993 Feb;28(2):121–122. doi: 10.1111/j.1600-0536.1993.tb03364.x. [DOI] [PubMed] [Google Scholar]
- Patel N. P., Patel K. H., Moster M. R., Spaeth G. L. Metipranolol-associated nongranulomatous anterior uveitis. Am J Ophthalmol. 1997 Jun;123(6):843–844. doi: 10.1016/s0002-9394(14)71139-0. [DOI] [PubMed] [Google Scholar]
- Perrenoud D., Bircher A., Hunziker T., Suter H., Bruckner-Tuderman L., Stäger J., Thürlimann W., Schmid P., Suard A., Hunziker N. Frequency of sensitization to 13 common preservatives in Switzerland. Swiss Contact Dermatitis Research Group. Contact Dermatitis. 1994 May;30(5):276–279. doi: 10.1111/j.1600-0536.1994.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Petounis A. D., Akritopoulos P. Influence of topical and systemic beta-blockers on tear production. Int Ophthalmol. 1989 Jan;13(1-2):75–80. doi: 10.1007/BF02028642. [DOI] [PubMed] [Google Scholar]
- Rudzki E., Kecik T., Rebandel P., Portacha L., Pauk M. Frequency of contact sensitivity to drugs and preservatives in patients with conjunctivitis. Contact Dermatitis. 1995 Oct;33(4):270–270. doi: 10.1111/j.1600-0536.1995.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T. M., Hodes B. L. Bilateral anterior uveitis associated with a brand of metipranolol. Arch Ophthalmol. 1997 Mar;115(3):421–422. doi: 10.1001/archopht.1997.01100150423020. [DOI] [PubMed] [Google Scholar]
- Weinreb R. N., Caldwell D. R., Goode S. M., Horwitz B. L., Laibovitz R., Shrader C. E., Stewart R. H., Williams A. T. A double-masked three-month comparison between 0.25% betaxolol suspension and 0.5% betaxolol ophthalmic solution. Am J Ophthalmol. 1990 Aug 15;110(2):189–192. doi: 10.1016/s0002-9394(14)76990-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. J., Baumann J. D., Hetherington J., Jr Side effects of timolol. Surv Ophthalmol. 1983 Dec;28 (Suppl):243–251. doi: 10.1016/0039-6257(83)90140-6. [DOI] [PubMed] [Google Scholar]