Abstract
Pineal glands removed from neonatal rats at 5, 7, and 9 days of age and explanted into short-term culture, synthesized melatonin when stimulated with norepinephrine (NE); their melatonin synthesis could not be suppressed with bright white light. Dispersed pineal cell cultures or pineal explants prepared from 1-day-old neonates and held in culture for 7 or 9 days also synthesized melatonin when stimulated with NE, but in these cases melatonin synthesis was significantly suppressed by light, demonstrating that the pineals had become photosensitive while in culture. The development of photosensitivity in culture could be partially or completely abolished by the continuous presence of 1 or 10 μm of NE in the culture medium. The pineals of all nonmammalian vertebrates are photoreceptive, whereas those of mammals do not normally respond to light. We hypothesize that a mechanism to suppress pineal photosensitivity by using NE released from sympathetic nerve endings evolved early in the history of mammals.
The photoreceptors that inform the circadian systems of vertebrates about environmental light cycles are surprisingly diverse. The circadian clocks of all nonmammalian vertebrates receive photic information from photoreceptors in several deep brain locations, as well as from the pineal organ, the parietal eye when it is present, as well as the retina. This photoreceptor extravagance disappears in mammals. Mammals use only retinal photoreceptors, albeit highly specialized ones, to perceive the day–night cycle (1). Elsewhere, we have argued that the selection pressure that produced this change was the need to adapt to the very different light environment in the nocturnal niche that the early mammals chose, in part to escape predation (2). Nocturnal animals, exposed to only very dim light, might receive conflicting messages from multiple photoreceptors with different thresholds, some of which might be above whereas others were below the ambient light intensity. In such an environment, mammals would be better off relying on their sensitive retinal circadian photoreceptors.
To effect this change, mammals had to either eliminate the extra retinal photoreceptive structures altogether or somehow render them unresponsive to light. In the special case of the pineal organ, it seems that the latter course was followed. Unlike pineal glands of all nonmammalian vertebrates, the adult mammalian pineal does not respond directly to light; like them, it still synthesizes melatonin rhythmically in synchrony with the light/dark cycle, but that cycle is perceived by photoreceptors in the retina and is transferred to the pineal by a multisynaptic neural pathway involving the suprachiasmatic nucleus of the hypothalamus and sympathetic input to the pineal itself (3).
Although the pineal of adult mammals does not respond to light, there is some indirect evidence for photoreception by the neonatal mammalian pineal. Furthermore, it is clear from both morphological and biochemical evidence that much of the photoreceptive machinery is still present in the pineal of neonatal mammals.
Optic enucleation of newborn rats does not prevent light regulation of pineal serotonin levels (4). Torres and Lytle (5, 6) showed that exposing blinded neonatal rats to light during the subjective night significantly reduced the activity of the melatonin-forming enzyme arylalkylamine N-acetyltransferase (AA-NAT). Sympathectomy of neonatal rat pineal does not interfere with light entrainment of pineal melatonin in the first weeks of life, whereas sympathectomy at later postnatal ages abolishes light entrainment (7, 8). However, because all these studies were carried out in whole animals, it has been argued that the effect of light may have been mediated by photosensitive structures other than the pineal itself (9, 10).
Many of the molecular components of the phototransduction pathway have been shown to be present in neonatal pineals. Rhodopsin immunoreactivity and rhodopsin mRNAs have been demonstrated in several mammalian species (11–16). Other data suggest the presence of cone cGMP phosphodiesterase in neonatal rat pineals (17), and a recent paper using in situ hybridization techniques has reported the presence in newborn rats and mice of many components necessary for functional phototransduction (18).
Pinealocytes of neonatal but not adult rats have photoreceptor-like morphology (19, 20). Furthermore, in vitro studies by Araki and colleagues (21–24) have shown that if neonatal rat pineals are dissociated and cultured, some cells express photoreceptor properties such as rhodopsin immunoreactivity and synthesis of rhodopsin transcript. The expression of these photoreceptive characteristics is inhibited by culturing dissociated pineals in the presence of norepinephrine (NE). In the studies reported here, we have extended the work of Araki and colleagues and have demonstrated photic control of melatonin synthesis in cultured pinealocytes and explants from neonatal rats.
Materials and Methods
Dispersed Cell Cultures.
Pineal glands (9–10/experiment) were obtained from neonatal Sprague–Dawley rats (within 24 h after delivery). They were placed in chilled Hanks' balanced salt solution (GIBCO/BRL) and treated for 30 min in 1 ml papain with DNase (Worthington) at 36°C. They were then triturated with glass fire-polished pipettes and added to an excess of culture medium consisting of DMEM without glutamate, with 10% newborn calf serum (NCS), B-27 supplements (GIBCO/BRL) and antibiotic, 100 units/ml penicillin, and 100 mg/ml streptomycin (GIBCO/BRL). This medium had previously been equilibrated with 5% CO2. The cells were centrifuged at 300 × g for 5 min and then resuspended in fresh medium. Aliquots of 50 μl were placed on 35-mm culture dishes, and the cells were allowed to attach for 10 min before the addition of 2 ml of DMEM with supplement but without NCS. The dishes were maintained in a 5% CO2 incubator at 36°C in darkness, unless otherwise specified, for the duration of the experiment. Medium was changed every 2 days. While the medium was being changed, the cultures were exposed briefly (<10 min) to light (<100 lux).
In the experiments in which cell cultures were continuously exposed to NE, medium containing 1 or 10 μM of NE was changed daily, and on the day of the experiment the medium was changed twice, at the beginning and the end of the 6-h period of light (or control dark) exposure.
Pineal Explant Cultures (Long Term).
Pineal glands (9–10/experiment) were obtained from neonatal rats within 24 h after birth and placed in chilled Hanks' balanced salt solution (GIBCO/BRL). Each pineal was then divided in half, and each half was transferred to a 35-mm culture dish. Pineals were allowed to attach for 10 min before the addition of 2 ml of DMEM with supplement but without newborn calf serum. The dishes were maintained in a 5% CO2 incubator at 36°C in darkness for the duration of the experiment, unless otherwise specified. Medium was changed every 2–3 days.
Pineal Explant Cultures (Short Term).
This procedure was identical to the previous one, except pineals were obtained from rats 5, 7, and 9 days old and were cultured for less than 1 day.
Light Delivery.
A fiber-optic light with a 150-W tungsten quartz–halogen lamp was used to illuminate the cultures. The light source was located outside the incubator, and the tip of the fiber-optic probe was inside the incubator about 15 cm from the plate containing the cultures. We estimated light intensities at the level of the cultures (2,000 μW/cm2) by placing the irradiance detector of a UDT 350 radiometer–photometer (United Detector Technology, Hawthorne, CA) at the same location as the culture plate. Although light exposure may have raised the temperature of the cultures by as much as 0.2°C, the limit of our ability to measure temperature, it is unlikely that the cultures were responding to temperature rather than light because the pineals explanted from 5-, 7-, and 9-day old rats were treated in the same way and did not respond (see Results).
Melatonin RIA.
Melatonin levels in the medium were measured by RIA for melatonin according to the methods of Rollag and Niswender (25) by using antibody kindly donated by Mark Rollag (Uniformed Service University, Department of Anatomy, Bethesda, MD 20814).
In Situ Hybridization.
Digoxigenin (DIG)-labeled antisense and sense cRNA probes for AA-NAT were synthesized by using a Genius4 DIG RNA labeling kit (Boehringer Mannheim). Template cDNA for the transcription was a 620-bp cDNA fragment of mouse AA-NAT subcloned into a pCRII vector (generously donated by Joseph Takahashi, Northwestern University, Department of Neurobiology and Physiology, Evanston, IL). We ascertained probe specificity by testing sense control probe for AA-NAT transcript on pineal cell cultures. No specific hybridization was seen. Our in situ hybridization protocol was modified from the method of Blackshaw and Snyder (18). For steps through the hybridization, all glassware was RNase free, and all solutions were diethyl pyrocarbonate treated and RNase free. Eighteen-millimeter-diameter coverslips containing cultured cells were postfixed for 20 min in 4% paraformaldehyde/PBS, washed 3 times for 3 min in PBS, and placed face down for 2 h in hybridization solution containing 50% formamide, 5× SSC, 5× Denhardt's solution, 500 mg/ml sonicated denatured herring sperm DNA, and 250 mg/ml Escherichia coli 600 tRNA. Coverslips were then transferred face down into hybridization solution containing 500 ng/ml probe, covered with a 22 × 60-mm glass coverslip, and hybridized overnight at 65°C. Large coverslips were removed by immersion in 5× SSC, and coverslips containing cells were washed 2 times for 1 h in 0.2× SSC at 65°C, and then for 5 min in Tris-buffered saline (TBS). Cells were then blocked in 10% heat-inactivated normal goat serum for 1 h at room temperature and incubated for 24 h at 4°C in this solution containing 1:5,000 dilution of sheep anti-DIG Fab fragments conjugated to alkaline phosphatase (Boehringer Mannheim). The next day, cells were washed 3 times for 5 min in TBS, one time for 5 min in buffer containing 0.1 M Tris, pH 9.5, 0.1 M NaCl, and 50 mM MgCl2, and then placed in a light-tight box in the same buffer containing 3.375 mg/ml nitroblue tetrazolium, 3.5 mg/ml 5-bromo-4-chloro-3-indoyl-phosphate, and 0.24 mg/ml levamisole. The color reaction was allowed to run for 3 days at room temperature and was then stopped in Tris/EDTA buffer and rinsed in ddH2O, and coverslips were mounted in Aquapolymount (Polysciences).
Results and Discussion
Melatonin Synthesis and Light Response in Pineal Cell Cultures.
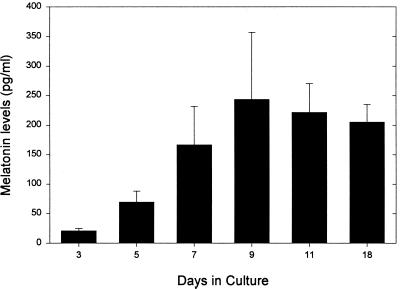
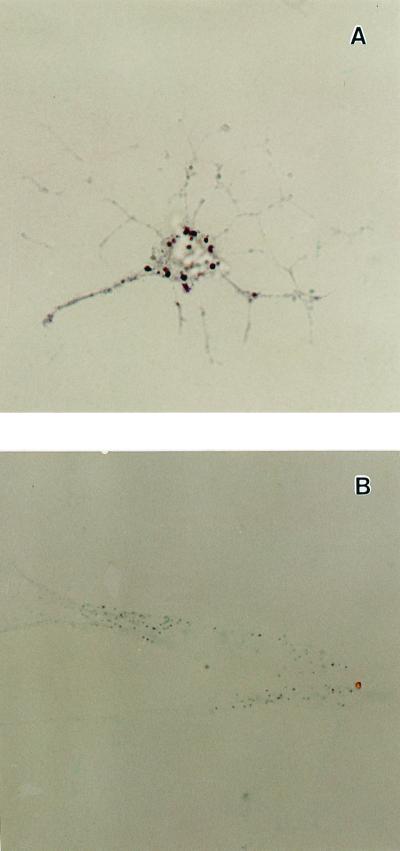
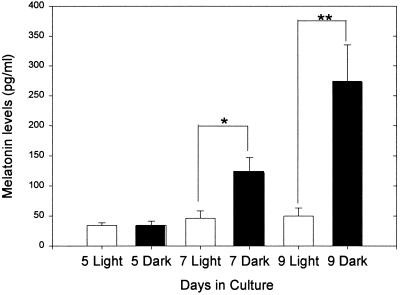
We prepared pineal cell cultures from 1-day-old neonatal rats and stimulated them with 10 μM of NE (experimental) or distilled H2O (control) for a 6-h period after 3, 5, 7, 9, or 11 days of culture and measured the melatonin produced. As expected (26, 27), dispersed cell cultures that were not stimulated with NE did not produce measurable amounts of melatonin, whereas dispersed cell cultures stimulated for 6 h with 10 μM of NE did synthesize melatonin in increasing amounts the longer they remained in culture before stimulation [up to 9 days (Fig. 1)]. Using in situ hybridization, we localized the mRNA for AA-NAT, the rate-limiting enzyme in melatonin synthesis (28, 29), in about 10% of the cells present in the stimulated culture. The message was localized in isolated cells that displayed a neuron-like morphology with numerous fine neuritic processes (Fig. 2). Interestingly, the shape of these cells was also very similar to the shape of opsin-immunoreactive cells in this type of pineal cell culture previously reported by Araki et al. (21). When dispersed cell cultures were stimulated for 6 h with 10 μM of NE and simultaneously exposed to white light (2,000 μWatt/cm2), the light significantly suppressed melatonin synthesis on days 7 and 9 of culture with respect to the levels measured in control cultures treated with NE but not exposed to light (Fig. 3).
Figure 1.
Melatonin levels ± SEM measured in dispersed pineal cell cultures (n = 10–12 cultures for each day). Cultures were obtained from postnatal day 1 rats and were maintained for 3, 5, 7, 9, 11, and 18 days before stimulation with NE. Melatonin levels were measured at the end of a 6-h period of stimulation with 10 μM of NE.
Figure 2.
Photomicrograph showing the expression of AA-NAT mRNA in pineal cells after 7 days in dispersed cell culture (magnification ×63). (A) Antisense probe localized message in isolated cells that displayed neuron-like morphology with numerous fine neuritic processes; (B) Sense probe (control) showed no significant labeling.
Figure 3.
Melatonin levels ± SEM measured in dispersed pineal cells cultured for 5, 7, and 9 days (n = 20–22 cultures for each treatment). Melatonin levels were measured at the end of a 6-h period of stimulation with 10 μM NE, during which the cells were exposed either to light (open bars) or dark (solid bars). ** denotes P < 0.01.
Melatonin Synthesis and Light Response in Pineal Explant Cultures (Long Term).
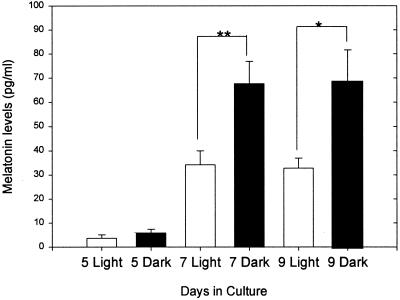
We repeated the experimental protocol described for dispersed pineal cell cultures on pineal explants obtained within 24 h of birth and cultured for 5, 7, and 9 days. The response of pineal explants after 5, 7, or 9 days of culture to stimulation with 10 μM of NE for 6 h in light or in dark was similar to the response of cultured pineal cells, although melatonin levels were lower (Fig. 4). There were significant decreases in melatonin synthesis in explants exposed to light after 7 and 9 days of culture.
Figure 4.
Melatonin levels ± SEM measured in pineal explants cultured for 5, 7, and 9 days (n = 19 cultures for each treatment). Melatonin levels were measured at the end of a 6-h period of stimulation with 10 μM NE, during which the explants were exposed either to light (open bars) or dark (solid bars). * denotes P < 0.05; ** P < 0.01.
Melatonin Synthesis and Light Response in Pineal Explant Cultures (Short Term).
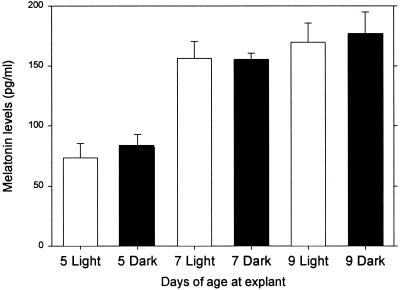
We cultured pineal explants from neonatal rats that were 5, 7, or 9 days old and asked whether they were light sensitive. Pineals were collected from rats at 5, 7, and 9 days of age and placed in culture. They were stimulated with 10 μM of NE for 6 h in either light or dark during the first 24 h of culture. In marked contrast to pineals removed from neonates and cultured for 7 or 9 days (Figs. 3 and 4), pineals removed on days 7 or 9 were not photosensitive (Fig. 5).
Figure 5.
Melatonin levels ± SEM measured in pineal explants obtained from neonatal rats at 5, 7, and 9 days of age (n = five cultures for each treatment). Melatonin levels were measured at the end of a 6-h period of stimulation with 10 μM NE, during which the explants were exposed either to light (open bars) or dark (solid bars). There were no significant differences between the light- and dark-exposed cultures.
Effect of Continuous Presence of NE on Melatonin Synthesis and Light Response in Dispersed Pineal Cultures.
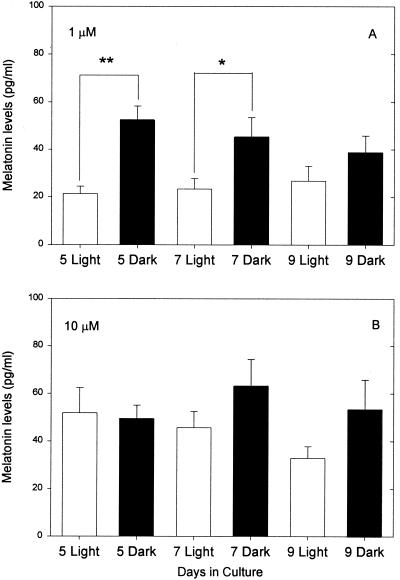
There are two general ways to interpret these data. Either prolonged culture actively induces photosensitivity, or removal of the pineal from a suppressive influence in vivo allows the development of photosensitivity. Given the work of Araki and colleagues and the fact that the pineals of all nonmammalian vertebrates are photosensitive, we favor the idea of suppression in vivo. We reasoned that NE itself released from sympathetic endings in the neonatal pineal might suppress the development of photosensitivity in the intact animal. To test this idea, we prepared pineal cell cultures from animals on day 1 as before and cultured them in the same medium used in previous experiments with either 1 μM or 10 μM of NE present continuously. We stimulated the cells with 10 μM of NE (see Materials and Methods) and exposed them to 6 h of light or dark after 5, 7, or 9 days in culture. Photosensitivity was suppressed by culture in 10 μM NE, whereas cells cultured with 1 μM of NE were photosensitive after 5 and 7 but not after 9 days in culture (Fig. 6). The overall amount of melatonin synthesized by these cultures was significantly lower than that produced by cells cultured without NE (Table 1). This is not surprising, because it has been shown previously that repeated administration of NE or isoproterenol produces subsensitivity in the response of NAT (27).
Figure 6.
Melatonin levels ± SEM measured in dispersed pineal cells cultured for 5, 7, and 9 days in medium containing (A) 1 μM NE and (B) 10 μM NE in fresh medium (see Materials and Methods; n = 16 cultures for each treatment). Melatonin levels in both cases were measured at the end of a 6-h period of stimulation with 10 μM NE, during which the cells were exposed either to light (open bars) or dark (solid bars). * denotes P < 0.05.
Table 1.
Melatonin levels in pg/ml in dispersed pineal cells cultured continuously with medium containing 1 or 10 μM of NE or without NE
| Culture | n | 5 days | n | 7 days | n | 9 days |
|---|---|---|---|---|---|---|
| Conditions | ||||||
| Without NE | 18 | 33.4 ± 8.1 | 18 | 131.1 ± 32.6 | 15 | 317.8 ± 82.3 |
| 1 μM NE | 16 | 52.2 ± 6.1 | 15 | 45.4 ± 8.1 | 14 | 38.6 ± 7.2 |
| 10 μM NE | 15 | 49.4 ± 5.7 | 15 | 61.5 ± 10.6 | 16 | 53.4 ± 12.3 |
Melatonin levels were measured at the end of a 6-h period of stimulation with fresh medium containing 10 μM of NE in the dark. Melatonin levels were significantly higher in cultures without NE at 7 and 9 days (t test, P < 0.05), but not at 5 days (n, number of cultures tested).
The lack of photosensitivity in the cells cultured in 10 μM of NE is not likely to be an artifactual result of the low levels of melatonin synthesis, because those levels were approximately the same in the cultures exposed to 1 μM that were photosensitive.
We can draw several conclusions from the results reported here. First, cultures prepared from the pineals of newborn rats are able to synthesize melatonin for more than 2 weeks. The cells begin to respond to 10 μM NE stimulation after 3 days in culture, and melatonin synthesis reaches a maximum between days 7 and 9 and then remains constant until at least day 18. These results are consistent with previous reports (26). Second, melatonin synthesis in these dispersed cell cultures (or in pineal explants) can be significantly suppressed by exposure to light on days 7 and 9 of culture. To our knowledge, this is the first direct documentation of photosensitivity in any mammalian pineal. Third, melatonin synthesis could not be suppressed by light in pineal explants obtained from rats aged 7 and 9 days and cultured for only 1 day.
These facts suggest to us that in the intact animal, something in the pineal's environment inhibits the development of a necessary component of the phototransduction cascade or its link to melatonin synthesis. Araki and collaborators (22–24) had shown that NE inhibits the photoreceptor-like morphology expressed by cultured cells from neonatal rat pineals. Furthermore, they demonstrated (24) the existence of a critical stage of sensitivity of cultured pineal cells to the effects of NE. Pineals removed later than postnatal day 6 contained only a very small number of cells that, when cultured, could differentiate into rhodopsin immunoreactive cells, whereas large numbers of cells differentiated in cultures obtained from neonatal rats on postnatal day 1 or 4. These results suggested that pineal cells may be sensitive to NE only until sometime between postnatal day 4 and 6, and that the effect of NE on differentiation is irreversible. Our data showing that pineals are not photosensitive when obtained from 5-, 7-, and 9-day-old rats that presumably have been exposed to NE in vivo are consistent with this interpretation (Fig. 5).
The work by Araki et al. (21–24) and our own results led us to the hypothesis that endogenous NE from sympathetic nerve endings in the pineal was a factor in suppressing pineal photosensitivity in mammals. We tested this idea by culturing dissociated pineal cells in the presence of constant amounts of NE (1 or 10 μM). The results lead to our fourth conclusion: the continuous presence of 10 μM NE inhibits development of pineal cell photosensitivity, whereas at 1 μM NE, photosensitivity was only partially suppressed. This result is partially in contrast to the work of Araki et al. (22–23), because they showed that NE inhibits rhodopsin immunoreactivity at much lower concentrations than 1 μM. There are at least two possible explanations of this apparent discrepancy: (i) rhodopsin immunoreactivity is suppressed by low concentration of NE, but enough rhodopsin remains to support the physiological response; (ii) the light response that we measure is mediated by a nonrhodopsin photopigment that is suppressed only by high levels of NE. The second explanation is not unreasonable, because a recent paper reported the presence of a blue cone photopigment in the neonatal rat pineal gland (18), and we have confirmed its presence in our photosensitive cultures with in situ techniques (S.D., G.T., and M.M., unpublished results).
Taken together, our results and those of others suggest that in the course of their early evolution, the mammals suppressed pineal photosensitivity by making relatively minor changes in the phototransduction machinery. Furthermore, the fact that neonatal mammalian pineals are more photoreceptor like than those of adults and may even be briefly photoreceptive suggests that the changes may have been made toward the end of the pathway that regulates the development of the pineal. Perhaps norepinehrine, which is the neurotransmitter that directly regulates melatonin synthesis in mammals, was coopted as the signal that initiates the changes that block the complete development of the pineal phototransduction pathway. Given these suggestions, it may be that the regulation of pineal photoreception is a particularly favorable situation in which to study the evolution of developmental mechanisms. It is in that context that we view the results reported here.
Acknowledgments
This work was supported by National Institutes of Health–National Institute of Neurological Disorders and Stroke Grants S-11NS38483–01 and U-54NS34194 to G.T. and by National Institutes of Health Grant MH56647 to M.M.
Abbreviations
- NE
norepinephrine
- AA-NAT
arylalkylamine N-acetyltransferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210248297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210248297
References
- 1.Freedman M S, Lucas R J, Soni B, Von Schantz M, Munoz M, David-Gray Z, Foster R G. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 2.Menaker M, Tosini G. In: Circadian Organization and Oscillatory Coupling. Honma K, Honma S, editors. Sapporo, Japan: Hokkaido Univ. Press; 1996. pp. 39–52. [Google Scholar]
- 3.Klein D C, Moore R Y, Reppert S M. Suprachiasmatic Nucleus: The Mind's Clock. New York: University Press; 1991. [Google Scholar]
- 4.Zweig M, Snyder S H, Axelrod J. Proc Natl Acad Sci USA. 1966;56:515–520. doi: 10.1073/pnas.56.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres G, Lytle L D. J Pineal Res. 1989;7:211–220. doi: 10.1111/j.1600-079x.1989.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 6.Torres G, Lytle L D. J Neural Transm. 1990;80:67–77. doi: 10.1007/BF01245023. [DOI] [PubMed] [Google Scholar]
- 7.Machado C R S, Machado A B M, Wragg L E. Endocrinology. 1969;85:846–848. doi: 10.1210/endo-85-5-846. [DOI] [PubMed] [Google Scholar]
- 8.Machado C R S, Wragg L E, Machado A B M. Science. 1969;164:442–443. doi: 10.1126/science.164.3878.442. [DOI] [PubMed] [Google Scholar]
- 9.Wetterberg L, Geller E, Yuwiler A. Science. 1970;167:884–885. doi: 10.1126/science.167.3919.884. [DOI] [PubMed] [Google Scholar]
- 10.Ulrich R, Yuwiler A, Wetterberg L, Klein D C. Neuroendocrinology. 1973;13:255–263. doi: 10.1159/000122210. [DOI] [PubMed] [Google Scholar]
- 11.Korf H W, Foster R G, Ekstrom P, Schalken J J. Cell Tissue Res. 1985;242:645–648. doi: 10.1007/BF00225432. [DOI] [PubMed] [Google Scholar]
- 12.Vigh-Teichmann I, Vigh B, Gery I, van Veen T. J Exp Biol. 1986;45:27–43. [PubMed] [Google Scholar]
- 13.Foster R G, Timmers A M, Schalken J J, de Grip W J. J Comp Physiol A. 1989;165:565–572. doi: 10.1007/BF00611242. [DOI] [PubMed] [Google Scholar]
- 14.Huang S K, Klein D C, Korf H W. Cell Tissue Res. 1992;67:493–498. doi: 10.1007/BF00319371. [DOI] [PubMed] [Google Scholar]
- 15.Kramm M, de Grip W J, Korf H W. Cell Tissue Res. 1993;274:71–78. doi: 10.1007/BF00327987. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Haeseleer F, Farriss R N, Huang J N, Baehr W, Milam A H, Palczewski K. Visual Neurosci. 1997;14:225–232. doi: 10.1017/s0952523800011366. [DOI] [PubMed] [Google Scholar]
- 17.Caracamo B, Hurwitz M Y, Craft C M, Hurwitz R L. J Neurochem. 1995;65:1085–1092. doi: 10.1046/j.1471-4159.1995.65031085.x. [DOI] [PubMed] [Google Scholar]
- 18.Blackshaw S, Snyder S H. J Neurosci. 1997;17:8074–8082. doi: 10.1523/JNEUROSCI.17-21-08074.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clabough J W. Am J Anat. 1973;137:215–230. doi: 10.1002/aja.1001370208. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman B L, Tso M O. J Cell Biol. 1975;66:60–75. doi: 10.1083/jcb.66.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araki M, Watanabe K, Tokunaga F, Nonaka T. Cell Differ Dev. 1988;25:155–164. doi: 10.1016/0922-3371(88)90008-1. [DOI] [PubMed] [Google Scholar]
- 22.Araki M, Tokunaga F. Cell Differ Dev. 1990;31:129–135. doi: 10.1016/0922-3371(90)90016-p. [DOI] [PubMed] [Google Scholar]
- 23.Araki M. Dev Biol. 1992;149:440–447. doi: 10.1016/0012-1606(92)90298-u. [DOI] [PubMed] [Google Scholar]
- 24.Araki M, Taketani S. Dev Brain Res. 1992;69:149–152. doi: 10.1016/0165-3806(92)90153-n. [DOI] [PubMed] [Google Scholar]
- 25.Rollag M, Niswender G D. Endocrinology. 1976;98:482–489. doi: 10.1210/endo-98-2-482. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman C M, Menaker M. J Pineal Res. 1991;11:173–178. doi: 10.1111/j.1600-079x.1991.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 27.Klein D C, Coon S L, Roseboom P H, Weller J L, Bernard M, Gastel J A, Zatz M, Iuvone P M, Rodriguez I R, Begay V, et al. Rec Prog Horm Res. 1997;52:307–358. [PubMed] [Google Scholar]
- 28.Borjigin J, Wang M M, Snyder S H. Nature (London) 1995;378:783–785. doi: 10.1038/378783a0. [DOI] [PubMed] [Google Scholar]
- 29.Coon S L, Roseboom P H, Baler R, Weller J L, Namboodiri M A A, Klein D C. Science. 1995;270:1681–1683. doi: 10.1126/science.270.5242.1681. [DOI] [PubMed] [Google Scholar]