Abstract
The selective subcellular localization of mRNAs to dendrites and the recent demonstration of local protein synthesis have highlighted the potential role of postsynaptic sites in modulation of cell–cell communication. We show that epitope-tagged subunit 2 of the ionotopic glutamate receptor, GluR2, mRNA transfected into isolated hippocampal neuronal dendrites is translated in response to pharmacologic stimulation. Further, confocal imaging of N-terminally labeled GluR2 reveals that the newly synthesized GluR2 protein can integrate into the dendritic membrane with the N terminus externally localized. These data demonstrate that integral membrane proteins can be synthesized in dendrites and can locally integrate into the cell membrane.
Glutamate receptors are integral membrane proteins that bind glutamate and provide the primary excitatory responsiveness of the central nervous system. Glutamate is involved in other aspects of central nervous system functioning, including modulation of development, whereas excessive glutamate functioning results in excitotoxicity. As an example of glutamate's involvement in early brain development, excitatory synaptic transmission is mediated by the actions of glutamate on N-methyl-D-aspartate (NMDA) receptors, as these synapses are thought to lack functional α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (silent synapses) (1). Alternative explanations for the mechanistic underpinnings of silent synapses include hypotheses such as synaptically localized AMPA receptors that are inactivated by posttranslational modifications (1) or neurotransmitter spillover (2, 3). These “AMPA-receptorless” synapses are electrophysiologically silent synapses, as synaptic transmission is not detected at resting membrane potential. In general as development progresses, there is a conversion of the silent synapse to an active state (1), although there is a population of potentially silent synapses containing NMDA receptors but lacking AMPA receptor subunits in the adult hippocampus as well (4, 5). The conversion of silent to active synaptic junctions has been postulated to be dependent on the acquisition of functional AMPA receptors (1). This is likely an important regulatory mechanism controlling aspects of synaptic plasticity in the developing brain that may persist into the adult. If acquisition of AMPA receptors by synapses occurs, then it may happen via four mechanisms: 1) activation of already present AMPA receptors, 2) the synthesis of the receptor in the soma and transport to the dendrites, 3) dendritic membrane insertion of preexisting intradendroplasmic pool of GluR2 protein (6), or 4) targeting of glutamate receptors to specific synapses after synthesis from dendritically localized glutamate receptor mRNAs. Although the first three possibilities likely play a role in elaborating the glutamate receptor repertoire in dendrites, we hypothesized that dendritic translation of glutamate receptor mRNAs and insertion of the newly translated glutamate receptor into the membrane may also contribute to the generation of the dendritic glutamate receptors. This possibility is supported by the recent experiments using a RNA amplification expression profiling with the PCR that has demonstrated the presence of ionotropic glutamate receptor mRNAs in neuronal (7–9) processes. Additionally, the mRNA for other integral membrane proteins has been found in dendrites including GABA-A receptors (8) and Ca2+ channels (8). Because dendritic protein synthesis has been shown to be required for long-term changes in synaptic efficacy (10, 11), dendritic translation and insertion of these receptors into the plasma membrane may be of functional significance.
We hypothesized that mRNAs encoding integral membrane proteins, such as neurotransmitter receptors, can be locally translated in dendrites and integrated into the cellular membrane. Data will be presented showing that cMyc fusion constructs with GluR2 subunit mRNA can indeed be translated in response to pharmacologic activation of metabotropic glutamate receptors in the dendrites of neurons from primary cell cultures of rat hippocampi, and these fusion proteins can be inserted into the plasma membrane. These results show that dendrites have the capacity to locally synthesize integral membrane proteins and to integrate them into the postsynaptic membrane, suggesting that a functional rough endoplasmic reticulum and Golgi apparatus (12) exist in this subcellular compartment. These results suggest a potential mechanism for the modulation of the receptor repertoire under specific synapses through altering the subunit representation locally in response to synaptic stimulation.
Methods
RNA Synthesis.
Two different GluR2 constructs were synthesized, one with a cMyc epitope on the C terminus and another with the cMyc epitope on the N terminus. DNA encoding the c-myc epitope EQKLISEEDL was introduced into GluR2 cDNA (M85035.1 GI:204381) using the PCR with the high fidelity polymerase PfuTurbo (Stratagene). The C-terminal c-myc construct was straightforwardly made by PCR extension of the cloned GluR2 cDNA. The N-terminal c-myc construct was more complicated with the c-Myc epitope inserted between the signal sequence and amino acid 1 of the mature GluR2. This was accomplished by PCR using the primer caacaagtcctcttcagaaatgagcttttgctcctcaccaaaaaatcagtcccataaaa to generate a 413-bp PCR product containing the GluR2 signal sequence followed by c-myc at its 3′ end. The primer gaggagcaaaagctcatttctgaagaggacttgttggtctcttctaacagcatacagataggg was used to generate a 3146-bp fragment of GluR2 containing the c-myc epitope at the 5′ end of the fragment preceding amino acid 1 of the mature GluR2. These two fragments were annealed using a second round of PCR (with primers to the 5′ and 3′ ends of GluR2) and then sequenced to check for spontaneous mutations. The c-myc epitope was encoded between the signal sequence and amino acid 1 of GluR2 protein for extracellular exposure to anti-cMyc 9E10 (Sigma) antibody at the N terminus of the receptor. These plasmid constructs were linearized with SpeI (GluR2–cMyc) and NotI (cMyc–GluR2), and to remove RNAses the plasmids were treated with proteinase K (100 μg/ml) and SDS (0.5%) at 50°C for 1 h. The plasmid was then phenol-chloroform extracted and ethanol precipitated. In vitro transcribed capped RNA was generated using Ambion's mMessage mMachine T7 RNA polymerase kit for GluR2-cMyc and SP6 RNA polymerase kit for cMyc-GluR2.
Cationic Liposome-Mediated RNA Transfection.
Primary dissociated neuronal cultures were generated from gestational day 18 embryonic rat hippocampi as previously described (7) and were grown on CELLocate microgrid coverslips (Eppendorf). These hippocampal neurons were cultured for 3 to 11 days in 35-mm Petri dishes containing physiologic saline solution (10 mM Hepes, pH 7.4/3 mM KCl/3 mM CaCl2/1.0 mM MgCl2/155 mM NaCl/1.0 mM glucose). Neuronal cell bodies were severed from their dendrites with a microelectrode and then aspirated, leaving the isolated dendrites adhered to the coverslip. Photomicrographs were taken and used to locate the transfected dendrites for light and confocal microscopic analysis. In early experiments, 5 μg of reporter RNA along with 5 μg of carrier tRNA was allowed to complex with 5 μg of cationic DOSPER liposomal transfection reagent [1,3-di-oleoyloxy-2-(6-carboxy-spermy)-propylamid; Boerhinger Mannheim] 15 min at room temperature. In later experiments, 2 μg of reporter RNA was complexed with 5 μl of GenePORTER transfection reagent (Gene Therapy Systems) for 30 min. The lipid–mRNA complex was applied directly onto severed dendrites with a microelectrode. [(RS)-3-5-dihydroxyphenylglycine] (DHPG, final 50–500 μM) was added to enhance protein synthesis (13, 15). This same transfection procedure was used to transfect both severed dendrites as well as intact neurons. The coverslips were then placed in the incubator for 30–120 min.
Immunohistochemistry.
Following RNA transfection, cells were washed in 1 × PBS. Cells were fixed in 4% paraformaldehyde, washed in 1 × PBS, followed by 1 × PBS with 0.1% H2O2, and preincubated in 5% normal goat serum with 0.1% Triton X-100. Cells were then labeled with the primary antibody, a monoclonal anti-cMyc (purchased either from the University of Pennsylvania Cell Center or from Sigma; 1:2000 dilution), or polyclonal anti-ATF-2 (New England Biolabs; 1:2500 dilution) overnight at 4°C. The avidin–biotin conjugation method was used to process the antibody staining (Vectastain ABC, Vector Laboratories). 3,3′–Diaminobenzidine was used to visualize immunoreactivity. For immunofluorescence, dendrites were washed in 1 × PBS following transfection and permeabilized during the exposure of the dendrites to the primary antibody (130 min) with 0.2% saponin, 5% normal goat serum in PBS. In parallel, isolated intact dendrites were preincubated in 5% normal goat serum/PBS in the absence of saponin. Dendrites were double-labeled with a 1:500 dilution of monoclonal anti-cMyc and a 1:2000 dilution of polyclonal anti-MAP2 (gift of C. Garner, University of Alabama, Birmingham). Cells were then washed in PBS and fixed in 2% paraformaldehyde. Cells were washed and labeled with anti-mouse FITC and anti-rabbit Texas Red (Jackson ImmunoResearch). Images were acquired with a Bio-Rad 1024 confocal microscope (University of Pennsylvania Pathology Department) mounted on a Nikon Eclipse E600 upright microscope using a 60×/0.14 oil lens (DIC H ∞/0.17 WD 0.21). A confocal optical section of 2.0 (from a confocal range of 0.7 to 8.0) was accumulated 20 times with 30% laser power.
Results
Transfection and Expression of GluR2–cMyc in Isolated Dendrites.
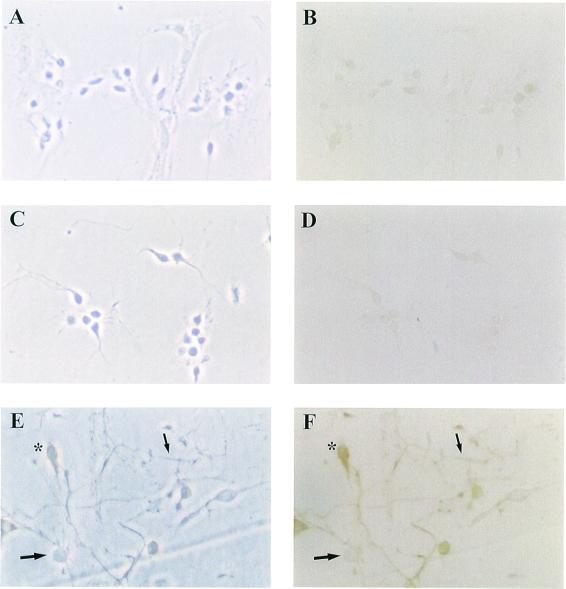
mRNA, to be used for the transfections, was synthesized from a PCR-generated fusion construct of GluR2 with the cMyc epitope sequence. Fig. 1 demonstrates the methodology used to determine whether the GluR2–cMyc fusion construct could be translated in primary cell cultures of rat hippocampal neurons cultured for 3 to 10 days (Fig. 2A) following transfection with lipid-encoated capped GluR2–cMyc mRNA. This protein synthesis assay was first used to prove that protein synthesis could occur in isolated dendrites (8). Local translation of the transfected mRNAs was assessed by immunohistochemistry using light microscopy to visualize the cMyc epitope tag present on the glutamate receptor protein made from the fusion construct mRNA. In Fig. 2F, immunoreactive cell bodies can be seen (asterisks). In addition, cell bodies were severed from their dendrites with a microelectrode and removed by aspiration, leaving the isolated dendrites adhered to the coverslip. After these isolated dendrites were transfected with GluR2–cMyc mRNA, they exhibited cMyc immunoreactivity, proving that glutamate receptor mRNAs can be translated in isolated dendrites (Fig. 2F, small arrow, and Fig. 3H, small arrow). Similar results have been found in cMyc fusion construct mRNA of NMDAR1 (data not shown). As controls, no immunoreactivity was detectable in the absence of transfected mRNA (Figs. 2B and 3D) or in transfected cells where the cMyc primary antibody is absent from the immunohistochemistry procedures (Fig. 2D). These results show that nonspecific immunoreactivity is not induced by the transfection protocol, secondary antibody, or immunohistochemical procedure.
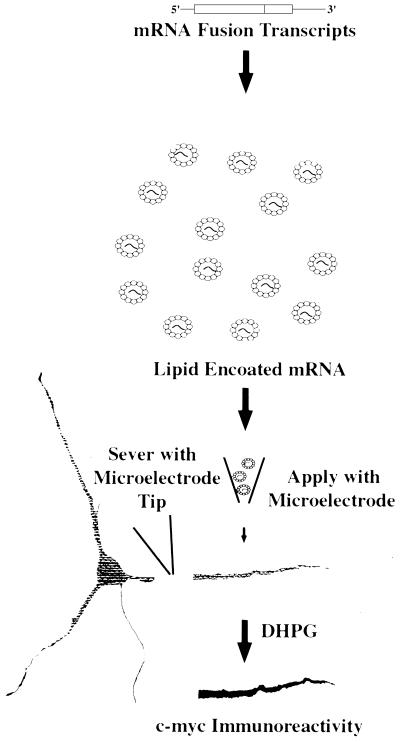
Figure 1.
Single dendrite transfection protocol. This schematic shows the experimental procedure for mRNA transfection of isolated dendrites. This assay was used to generate the data in the subsequent figures.
Figure 2.
Epitope-tagged GluR2–cMyc mRNA transfection into cells and single dendrites. A, C, and E contain phase contrast photographs of cells in B, D, and F, respectively. (B) Immunohistochemical background staining of hippocampal neurons that have not been transfected with the fusion mRNA construct but have been taken through the immunohistochemical procedure. (D) This photomicrograph shows the immunohistochemcial background staining of cells that had been transfected with the fusion construct followed by immunohistochemical staining in the absence of cMyc primary antibody. (F) Immunohistochemical proof of local protein synthesis of the transfected reporter construct mRNA in single, isolated dendrites and whole cells. Also visible in this field of cells are some cells that were not transfected. All of the cultures were treated with DHPG. *, transfected cell body; large arrow, untransfected cell; small arrow, transfected dendrite.
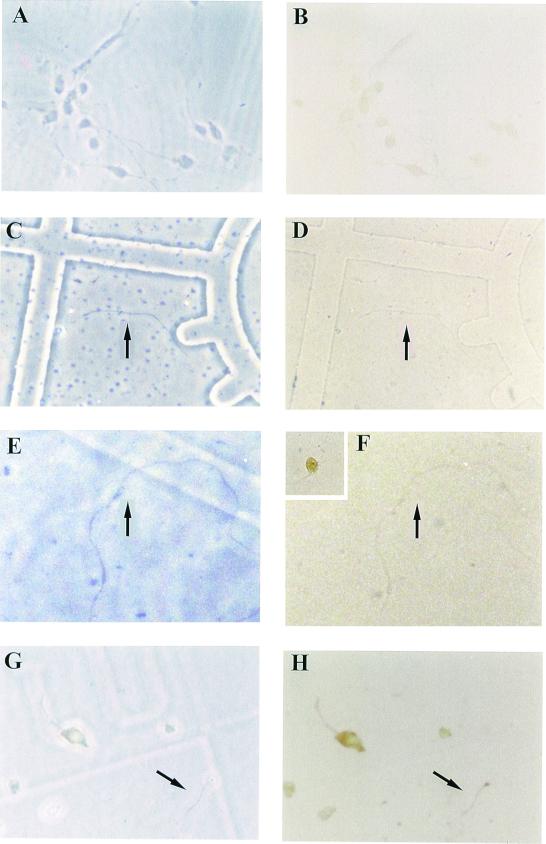
Figure 3.
Metabotrobic glutamate receptor activation of GluR2–cMyc protein translation. A, C, E, and G are phase contrast photographs of cell fields that correspond to B, D, F, and H, respectively. (B) These cells show that transfection of GluR2–cMyc fusion construct mRNA into cells, in the absence of DHPG stimulation, results in minimal translated protein. (D) This nontransfected dendritic process is stained with the cMyc antibody and shows no immunoreactivity. (F) This nontransfected dendrite is stained with an ATF-2 antibody and exhibits no immunostaining. The Inset in the upper left corner shows a stained neuron from the same culture, thus showing that ATF-2 is present in the neuronal cell body but is absent from dendrites. (H) GluR2–cMyc immunoreactivity generated after transfection of the GluR2–cMyc fusion construct mRNA that had been stimulated by the addition of 500 μM DHPG into the media. Arrow points to the individual isolated dendrite described herein.
Metabotropic Glutamate Receptor Activation of GluR2–cMyc Translation.
In Fig. 2, cMyc immunoreactivity was detectable because local dendritic protein synthesis was stimulated by DHPG, a metabotropic glutamate receptor agonist. DHPG stimulates protein synthesis by stimulating translocation of p90rsk to polyribosomes, which in turn phosphorylates proteins associated with the translational complex allowing for more ribosome initiation and translocation (13). Additionally, metabotrobic receptor activation stimulates calcium waves throughout the dendrite (14), which is likely an important regulator of dendritic protein synthesis through modulation of the endoplasmic reticulum. The stimulation paradigm used in this study was incubation of the cultures with DHPG, which has been shown to be effective in stimulating dendritic protein synthesis using synaptoneurosome preparations (13, 15) as well as the isolated dendrite preparation (16). In our studies, DHPG (50–500 μM) was incubated with the dendrites or intact cells for 30 or 120 min (13, 15). Data presented in Fig. 3 demonstrate that cells transfected with the GluR2–cMyc mRNA yield differential amounts of immunoreactivity dependent upon whether or not the cells were incubated with DHPG after the transfection. In the absence of DHPG there is little immunoreactivity in transfected cells (Fig. 3B). As additional controls to ensure that proteins are not being indiscriminately induced by the experimental procedure (dendrite severing or DHPG treatment), we looked for c-myc immunoreactivity in isolated dendrites that had not been transfected and found no c-myc immunoreactivity (Fig. 3D); using an ATF-2 transcription factor antibody (17), we show that ATF-2 protein is present in the neuronal cell soma and nucleus (Fig. 3F Inset) but is absent from dendrites still connected to the cell body (Fig. 3F Inset) as well as from isolated dendrites (Fig. 3F). This ATF-2 experiment serves as a negative control highlighting the specificity of regulated glutamate receptor synthesis in the dendrite. Following treatment with DHPG, there is a dramatic increase in immunoreactivity observed within the cell bodies as well as in dendrites severed before the transfection protocol (Fig. 3H, arrow). These data show that DHPG can stimulate GluR2–cMyc synthesis in both cell bodies and isolated dendrites.
Membrane Expression of N-Terminally Labeled GluR2.
To determine whether GluR2, newly synthesized in the isolated dendrite, can be inserted into the membrane (a requirement for receptor functionality), we used permeabilized (showing membrane and dendroplasm localization) versus intact (showing only cell surface localization) dendrite preparations. Briefly, we made an N-terminally epitope-tagged GluR2 cDNA construct that upon translation of its mRNA would give rise to a GluR2 receptor subunit with the c-myc epitope tag on the N terminus of the protein. The N terminus of AMPA glutamate receptors has been shown to be on the external surface of the cellular membrane (18). Consequently, if the N-terminally localized c-myc–GluR2 is detectable on intact dendrites, then the c-Myc–GluR2 protein must have been integrated into the dendritic plasma membrane with the c-Myc N terminus extracellular. To control for this extracellular localization and to ensure that the plasma membrane remains intact, we tested the integrity of the plasma membrane by looking for the presence of Map2, a highly abundant protein that is localized in the cytoplasm of dendrites.
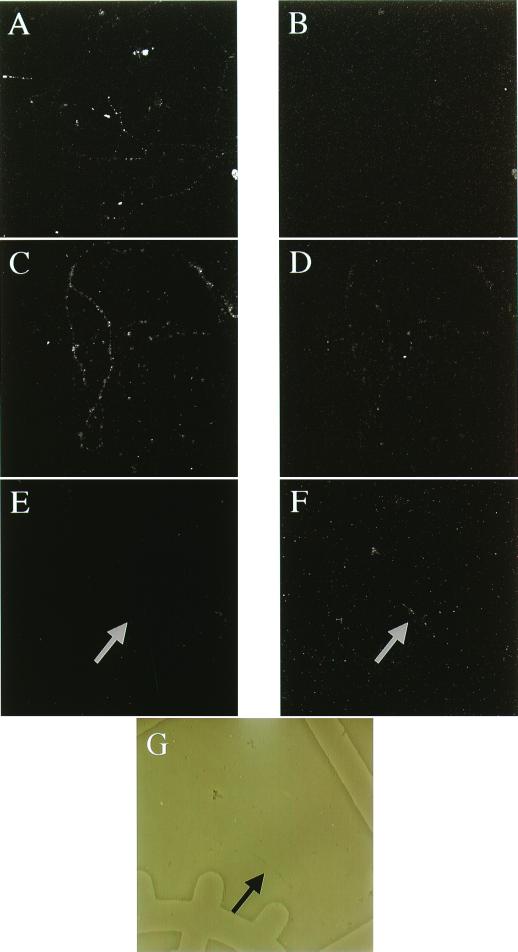
In Fig. 4 data are presented that show intact and permeabilized dendrites in which endogenous Map2 and c-Myc–GluR2 translated from transfected RNA are examined. In Fig. 4 A and B, permeabilized dendrites that have been mock transfected (transfection procedure minus the cMyc–GluR2 mRNA) show immunofluorescence staining with Map2 polyclonal serum (A) and absence of cMyc staining (B). In Fig. 4A, a strong signal is apparent consistent with the cytoplasmic localization of Map2 protein in dendrites. Fig. 4B is a negative control illustrating the absence of c-Myc immunoreactivity in dendrites that have not been transfected with the reporter construct. Immunohistochemical detection of cytoplasmically localized Map2 and protein translated from transfected c-Myc-GluR2 is shown in Fig. 4 C (Map2) and D (c-Myc-GluR2). Nonpermeabilized dendritic membranes show an absence of Map2 staining (Fig. 4E), whereas cMyc–GluR2 is readily detectable in the plasma membrane of these nonpermeabilized dendrites as shown in Fig. 4F. The absence of Map2 staining in intact dendrites coupled with the presence of membrane staining for c-Myc–GluR2 shows that this dendritically synthesized glutamate receptor subunit can be integrated into the plasma membrane of the dendrite.
Figure 4.
Membrane localization of dendritically synthesized c-Myc–GluR2. A, C, and E show immunofluorescence of MAP-2, whereas B, D, and F show the double-labeled anti-cMyc immunofluorescence in the same dendrites. G shows a phase contrast photomicrograph of the dendrite shown in E and F. (A and B) Dendrites that have been permeabilized and not transfected with reporter RNA. (C and D) Dendrites that have been permeabilized and transfected. (E–G) Intact cells that have been transfected with reporter RNA.
Discussion
Protein synthesis in dendrites is extensively described, yet the translation of integral membrane proteins in this subcellular domain has been problematic. There is clear evidence of smooth endoplasmic reticulum in dendrites, but the presence of rough endoplasmic reticulum (RER) and Golgi apparatus has been controversial. The presence of membrane proteins such as receptors in dendrites was believed to occur by transport of the protein from the soma to the dendrites, where they are concentrated in synaptic areas (19). We have shown that integral membrane proteins can be synthesized in dendrites and inserted into the membrane, suggesting that there is a functional RER and Golgi in the dendrite. Additionally, presuming that the traditional pathway for membrane protein integration into membranes is used, it is intriguing that mRNA transfected into the dendrite can associate with the RER. This suggests that the protein components necessary for sorting of the mRNA to RER and through the Golgi are present in the dendrite and able to freely interact with mRNAs. At least some of these proteins, including SRP68 and BiP (both of which are involved in protein recognition by the Golgi), have been identified in the dendrite using immunohistochemical procedures (20, 21). Additionally, there is evidence that N-linked glycosylation can occur in dendrites (22), again suggesting that there is a regionally active Golgi.
Alternatively, a novel mechanism for the insertion of newly synthesized membrane proteins into the cell membrane cannot be discounted. There is precedent for a novel Golgi complex at the neuromuscular junction (23). Using antibodies to various components of the RER and Golgi complex, it was shown, in the proximity of the motor endplate, that the RER was distributed throughout the myofibers, whereas the Golgi complex was compartmentalized in the subneural region. This subneural Golgi complex was distinct from other Golgi complexes in showing a distinct pattern of immunoreactivity with some standard Golgi epitopes being present (a small GTP-binding protein rab6p and a 160-kDa sialoglycoprotein), whereas others were absent (TGN38 and alpha-mannosidase II). This specialization suggests that the subneural Golgi complex is different from Golgi compartmentalized to other regions of the myofiber. The single dendrite transfection assay and the use of fusion constructs should allow us to further investigate the biochemistry of dendritic protein synthesis and integral membrane protein insertion into membranes.
The requirement for pharmacologic stimulation of dendritic protein synthesis (Fig. 3 B and H) suggests that mRNAs localized in dendrites await appropriate stimulation as a condition for translation to occur. The localization of mRNAs in dendrites and their translation response to pharmacologic or synaptic stimulation can result in proteins that can act locally (as shown herein) or distal to the dendrites (16). This stimulus-dependent local protein synthesis may be one of the critical reasons for the partitioning of mRNAs to this subcellular compartment.
The confocal microscopy studies of the transfected dendrites show that cMyc–GluR2 was integrated into the plasma membrane with the c-Myc epitope exposed to the extracellular solution. Data in Fig. 3 show that cytoplasmic c-Myc-labeled GluR2 is visible after 30 min of DHPG stimulation. In the studies to examine membrane localization of dendritically synthesized c-Myc–GluR2, we incubated the transfected dendrites for 120 min to ensure that various translation and posttranslational processing events necessary to produce a membrane receptor have been completed. The present studies do not permit us to quantitate differences in DHPG-stimulated rates of glutamate receptor synthesis versus insertion of the receptor into the membrane.
The local synthesis and membrane insertion of glutamate receptors in dendrites has implications for many aspects of the neuronal functioning. For example, it has been postulated that long-term potentiation (LTP) requires postsynaptic neuronal exocytosis (24). One mechanism that would be consistent with this is the transport of glutamate receptors to the membrane through the secretory pathway. Additionally, the work of Kang and Schuman (10), suggesting that stimulated protein synthesis in dendrites is necessary to develop LTP, is consistent with and supported by the idea that functional ionotropic glutamate receptors can be made in dendrites and function locally to modulate the responsiveness of individual synapses. Interestingly, the concept of the Hebbian synapse (in which particular synapses can be reinforced by an increase in stimulation from the presynaptic neuron that is regulated by the postsynaptic neuron) led to the idea that memories can be distributed throughout neuronal networks through the strengthening of particular neuronal connections (25). This distribution hypothesis coupled with the more recent discovery that dramatic dendritic restructuring occurs during learning (26) suggests that dendrites may be the important cellular components regulating the acquisition and retention of memories. Based on the biochemical data presented herein, it is reasonable to speculate that dendrites can be subdivided into different synaptically defined subregions in which individual synapses are differentially responsive to glutamate by virtue of which glutamate receptor mRNAs are targeted to individual synapses, the rate of stimulated glutamate receptor synthesis and insertion into the membrane, and the functional responsiveness of these newly synthesized glutamate receptors as modulated by the second messenger systems and interacting proteins that are locally present within the individual synaptic areas. These newly synthesized receptors working in concert with the receptors that are present in the synapse before stimulation of new receptor production would modulate the glutamate responsiveness of the synapse. This “receptor repertoire” model adds a potentially important corollary to the modified Hebbian synapse model described above and provides a mechanism for differentially distributing and strengthening glutamate-activated synapses along an individual dendrite.
Another implication of the findings presented herein, as discussed earlier, silent synapses are thought to occur by virtue of the synapses exhibiting NMDA receptor responsiveness with little to no AMPA responsiveness. This AMPA responsiveness develops with time and has been postulated to occur via recruitment of glutamate receptors to these previously silent synapses. Indeed, the insertion of locally synthesized receptors into silent synapses may confer AMPA responsiveness to these synapses. In this example as well as the LTP model presented above, synaptic stimulation is required for either LTP or development of AMPA responsiveness to occur just as it is needed for local dendritic protein synthesis to occur.
Discovery of the stimulated synthesis and membrane insertion of integral membrane proteins in the dendrite raises several interesting possibilities. These data suggest that the glutamate responsiveness of synapses may be inextricably linked to the pool of neurotransmitters in the synaptic cleft. Indeed, if a particular neurotransmitter or growth factor, such as glutamate, brain-derived neurotrophic factor, or NT3 (8, 10), can stimulate local receptor synthesis, then synapses that are responsive to these signaling molecules may be able to alter their local receptor repertoire. Glutamate responsiveness may also be altered in that newly synthesized receptors may have a distinct set of posttranslational modifications as well as unique associations with accessory proteins (e.g., PSD 95, GRIP) from these receptors that have been present in the dendrite for longer periods of time. Dendritically synthesized membrane receptors may be modified by selective second messenger systems that are enriched in the synaptic microenvironment. Hence, in addition to supplementing the already extant receptors, those with unique functional properties may be generated. Regardless, the local synthesis and membrane insertion of glutamate receptors in dendrites suggests a mechanism for the conversion of silent to active synapses as well as other dynamic shifts in neuronal functioning that could follow from local modification of the molecular constituents of glutamate receptor-mediated excitatory synapses (24, 27).
Acknowledgments
The efforts of Margie Price in preparing the hippocampal cultures are greatly appreciated. We thank C. Garner for the Map2 polyclonal antibody. We thank K. Miyashiro for helpful discussions. This work was supported by National Institutes of Health Grants AG9900 and MH58561 (to J.E.).
Abbreviations
- RER
rough endoplasmic reticulum
- LTP
long-term potentiation
- DHPG
[(RS)-3-5-dihydroxyphenylglycine]
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
N-methyl-d-aspartate
References
- 1.Petralia R S, Esteban J, Wang Y-X, Partridge J, Zhao H-M, Wenthold R. Nature Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- 2.Asztely F, Erdemli G, Kullmann D M. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 3.Rusakov D A, Kullmann D M. J Neurosci. 1998;18:3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y, Janssen W G, Morrison J H. J Neurosci Res. 1998;54:444–449. doi: 10.1002/(SICI)1097-4547(19981115)54:4<444::AID-JNR2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Nusser C, Lujan R, Laube G, Roberts J D, Molnar E, Somogyi P. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 6.He Y, Janssen W, Vissavajjhala P, Morrison J H. Exp Neurol. 1998;150:1–13. doi: 10.1006/exnr.1997.6720. [DOI] [PubMed] [Google Scholar]
- 7.Miyashiro K, Dichter M, Eberwine J. Proc Natl Acad Sci USA. 1994;91:10800–10804. doi: 10.1073/pnas.91.23.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crino P, Eberwine J. Neuron. 1996;17:1173–1187. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 9.Gazzaley A, Benson D, Huntley G, Morrison J. J Neurosci. 1997;17:2006–2017. doi: 10.1523/JNEUROSCI.17-06-02006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang H, Schuman E. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 11.Liu S-Q J, Cull-Candy S G. Nature (London) 2000;405:454–457. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- 12.Pelham H, Munro S. Cell. 1993;75:603–605. doi: 10.1016/0092-8674(93)90479-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angenstein F, Greenough W, Weiler I J. Proc Natl Acad Sci USA. 1998;95:15078–15083. doi: 10.1073/pnas.95.25.15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe D B, Brown T H. J Neurophysiol. 1994;72:471–474. doi: 10.1152/jn.1994.72.1.471. [DOI] [PubMed] [Google Scholar]
- 15.Weiler I J, Greenough W T. Proc Natl Acad Sci USA. 1993;90:7168–7171. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crino P, Khodakhah K, Becker K, Ginsberg S, Hemby S, Eberwine J. Proc Natl Acad Sci USA. 1998;95:2313–2318. doi: 10.1073/pnas.95.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Campbell D, Derijard B, Davis R. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 18.Bennett J A, Dingledine R. Neuron. 1995;14:373–384. doi: 10.1016/0896-6273(95)90293-7. [DOI] [PubMed] [Google Scholar]
- 19.Popov S, Poo M-M. J Neurosci. 1992;12:77–85. doi: 10.1523/JNEUROSCI.12-01-00077.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiedge H, Brosius J. J Neurosci. 1996;16:7171–7181. doi: 10.1523/JNEUROSCI.16-22-07171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardiol A, Racca C, Triller A. J Neurosci. 1999;19:168–179. doi: 10.1523/JNEUROSCI.19-01-00168.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torre E, Steward O. J Neurosci. 1996;16:5967–5978. doi: 10.1523/JNEUROSCI.16-19-05967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jasmin B, Antony C, Changuex J, Cartaud J. Eur J Neurosci. 1995;7:470–479. doi: 10.1111/j.1460-9568.1995.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 24.Lledo P, Zhang X, Sudhof T, Malenka R, Nicoll R. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 25.Zador A M, Koch C, Brown T H. Proc Natl Acad Sci USA. 1990;87:6718–6722. doi: 10.1073/pnas.87.17.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiler I J, Hawrylak N, Greenough W T. Behav Brain Res. 1995;66:1–6. doi: 10.1016/0166-4328(94)00116-w. [DOI] [PubMed] [Google Scholar]
- 27.Weiler I J, Wang X, Greenough W T. Prog Brain Res. 1994;100:189–194. doi: 10.1016/s0079-6123(08)60785-2. [DOI] [PubMed] [Google Scholar]