Abstract
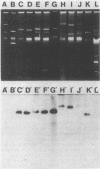
A total of 225 isolates of Campylobacter jejuni and 54 isolates of Campylobacter coli were screened for resistance to kanamycin. Among these, five resistant isolates of C. jejuni and six resistant isolates of C. coli, all with different plasmid patterns, were identified. Each contained at least one plasmid greater than or equal to 41 kilobases in size. The MIC of kanamycin for all 11 strains was determined to be greater than or equal to 256 micrograms/ml by an agar dilution method. In addition, all of the strains exhibited resistance to tetracycline (greater than or equal to 16 micrograms/ml). Eight of the 11 strains transferred the kanamycin resistance phenotype to other Campylobacter strains by conjugation. DNA from 9 of the 11 strains hybridized to a DNA probe specific for the 3'-O-aminoglycoside phosphotransferase type III gene. The remaining two strains also failed to show homology with DNA probes specific for the genes encoding 3'-O-aminoglycoside phosphotransferase types I, II, and III. The novel kanamycin resistance gene was cloned into the vector pBR322 and was expressed in Escherichia coli. Phosphocellulose paper binding assays on sonicates of the E. coli strain carrying the cloned kanamycin determinant demonstrated significant activity against kanamycin, neomycin, and amikacin but not against butirosin, gentamicin, tobramycin, or lividomycin, suggesting that the enzyme is the product of a 3'-O-aminoglycoside phosphotransferase type of aminoglycoside resistance gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Davies J. Plasmid-medicated aminoglycoside phosphotransferase of broad substrate range that phosphorylates amikacin. Antimicrob Agents Chemother. 1977 Apr;11(4):619–624. doi: 10.1128/aac.11.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Tenover F. C., Young S. A., Gordon K. P., Plorde J. J. Comparison of three DNA hybridization methods for detection of the aminoglycoside 2"-O-adenylyltransferase gene in clinical bacterial isolates. Antimicrob Agents Chemother. 1985 Jul;28(1):69–73. doi: 10.1128/aac.28.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Harris N. V., Thompson D., Martin D. C., Nolan C. M. A survey of Campylobacter and other bacterial contaminants of pre-market chicken and retail poultry and meats, King County, Washington. Am J Public Health. 1986 Apr;76(4):401–406. doi: 10.2105/ajph.76.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N. V., Weiss N. S., Nolan C. M. The role of poultry and meats in the etiology of Campylobacter jejuni/coli enteritis. Am J Public Health. 1986 Apr;76(4):407–411. doi: 10.2105/ajph.76.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Merriwether T. L., Tkalcevic G. T., Gemski P. Genetic studies of kanamycin resistance in Campylobacter jejuni. Antimicrob Agents Chemother. 1986 Aug;30(2):225–230. doi: 10.1128/aac.30.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Gerbaud G., Lambert T., Courvalin P. Acquisition by a Campylobacter-like strain of aphA-1, a kanamycin resistance determinant from members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1987 Jul;31(7):1021–1026. doi: 10.1128/aac.31.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M. J., Castillo J., Martin C., Navarro M., Gomez-Lus R. Aminoglycoside-phosphotransferases APH(3')-IV and APH(3") synthesized by a strain of Campylobacter coli. J Antimicrob Chemother. 1986 Aug;18(2):153–158. doi: 10.1093/jac/18.2.153. [DOI] [PubMed] [Google Scholar]
- Sagara H., Mochizuki A., Okamura N., Nakaya R. Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli with special reference to plasmid profiles of Japanese clinical isolates. Antimicrob Agents Chemother. 1987 May;31(5):713–719. doi: 10.1128/aac.31.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Garner R. S., Allan B. J. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1983 Dec;24(6):930–935. doi: 10.1128/aac.24.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., LeBlanc D. J., Elvrum P. Cloning and expression of a tetracycline resistance determinant from Campylobacter jejuni in Escherichia coli. Antimicrob Agents Chemother. 1987 Sep;31(9):1301–1306. doi: 10.1128/aac.31.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Williams S., Gordon K. P., Nolan C., Plorde J. J. Survey of plasmids and resistance factors in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1985 Jan;27(1):37–41. doi: 10.1128/aac.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Evolution and transfer of aminoglycoside resistance genes under natural conditions. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):93–102. doi: 10.1093/jac/18.supplement_c.93. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983 Sep;23(3):331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Gerbaud G., Lambert T., Courvalin P. In vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 1985 Dec 16;4(13A):3583–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. A., Tenover F. C., Gootz T. D., Gordon K. P., Plorde J. J. Development of two DNA probes for differentiating the structural genes of subclasses I and II of the aminoglycoside-modifying enzyme 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1985 May;27(5):739–744. doi: 10.1128/aac.27.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]