Abstract
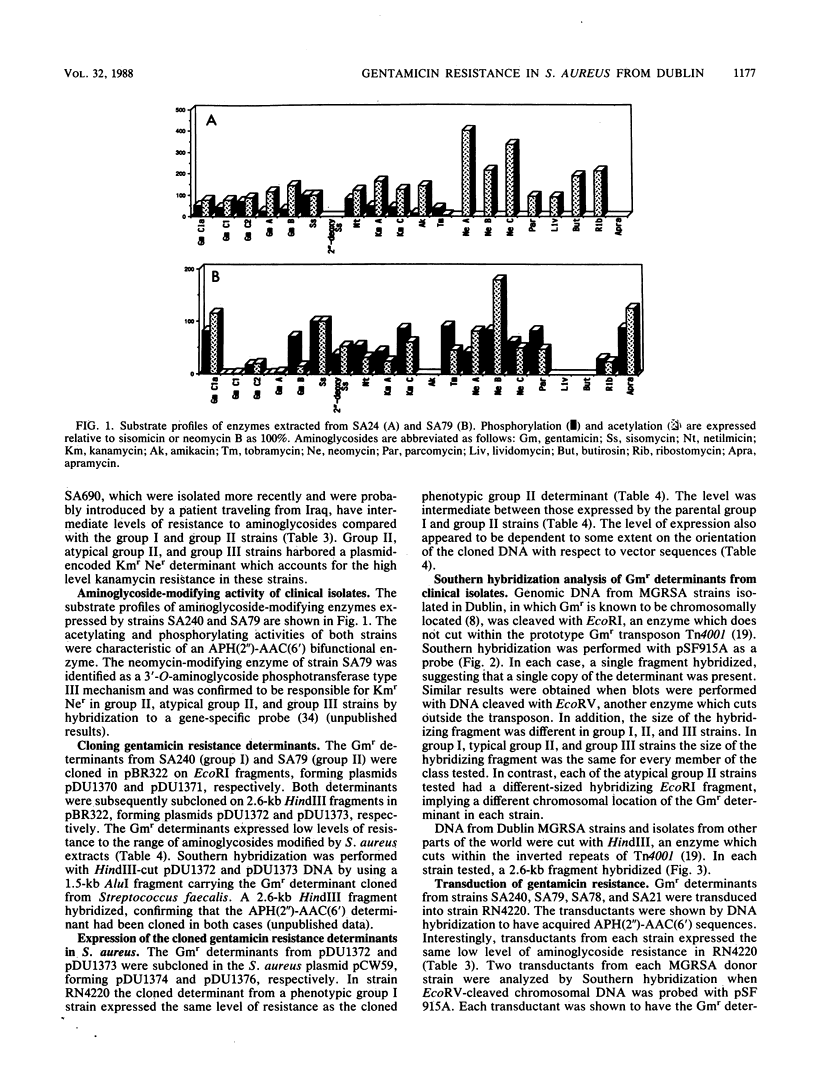
Methicillin- and gentamicin-resistant strains of Staphylococcus aureus isolated in Dublin hospitals have been classified into groups I, II, and III based on resistance to antimicrobial agents and plasmid profiles. Each group expresses a characteristic level of resistance to gentamicin, tobramycin, and sisomicin. Enzyme assays showed that resistant strains expressed 2"-aminoglycoside phosphotransferase-6'-aminoglycoside transferase activity by a determinant which is known to be chromosomally located. The gentamicin resistance (Gmr) determinants were transferred from group I, II, or III strains by transduction into a laboratory strain where each expressed the same low level of resistance. This finding suggests that high-level resistance in some clinical strains is due to a second, unlinked resistance mechanism. No evidence was obtained by hybridization experiments that clinical isolates or spontaneous mutants expressing high-level Gmr carried more than one copy of the Gmr determinant, thus eliminating the possibility that a gene dosage effect was responsible for high-level resistance. Hybridization experiments with transductants and wild strains suggested that the Gmr determinant was located at homologous sites in wild strains from different groups, although restriction site differences were observed in flanking sequences. Electron microscope analysis of a cloned Gmr determinant and genetic evidence suggested that a Dublin clinical isolate harbored a transposon very similar to Tn4001.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Coughter J. P., Johnston J. L. Plasmid-encoded trimethoprim resistance in staphylococci. Antimicrob Agents Chemother. 1986 May;29(5):733–740. doi: 10.1128/aac.29.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Johnston J. L. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob Agents Chemother. 1983 Jul;24(1):70–77. doi: 10.1128/aac.24.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asheshov E. H. Loss of antibiotic resistance in Staphylococcus aureus resulting from growth at high temperature. J Gen Microbiol. 1966 Mar;42(3):403–410. doi: 10.1099/00221287-42-3-403. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cafferkey M. T., Hone R., Falkiner F. R., Keane C. T., Pomeroy H. Gentamicin and methicillin resistant Staphylococcus aureus in Dublin hospitals: clinical and laboratory studies. J Med Microbiol. 1983 May;16(2):117–127. doi: 10.1099/00222615-16-2-117. [DOI] [PubMed] [Google Scholar]
- Coleman D. C., Pomeroy H., Estridge J. K., Keane C. T., Cafferkey M. T., Hone R., Foster T. J. Susceptibility to antimicrobial agents and analysis of plasmids in gentamicin- and methicillin-resistant Staphylococcus aureus from Dublin hospitals. J Med Microbiol. 1985 Oct;20(2):157–167. doi: 10.1099/00222615-20-2-157. [DOI] [PubMed] [Google Scholar]
- Coleman D., Falkiner F. R., Carr M. E., Dowd G., Dougan G., Keane C. T. Simultaneous outbreaks of infection due to Serratia marcescens in a general hospital. J Hosp Infect. 1984 Sep;5(3):270–282. doi: 10.1016/0195-6701(84)90076-8. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Davies J. Plasmid-medicated aminoglycoside phosphotransferase of broad substrate range that phosphorylates amikacin. Antimicrob Agents Chemother. 1977 Apr;11(4):619–624. doi: 10.1128/aac.11.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Solh N., Fouace J. M., Pillet J., Chabbert Y. A. Plasmid DNA content of multiresistant Staphylococcus aureus strains. Ann Microbiol (Paris) 1981 Sep-Oct;132B(2):131–156. [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Ruff E. A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Jaffe H. W., Sweeney H. M., Nathan C., Weinstein R. A., Kabins S. A., Cohen S. Identity and interspecific transfer of gentamicin-resistance plasmids in Staphylococcus aureus and Staphylococcus epidermidis. J Infect Dis. 1980 Jun;141(6):738–747. doi: 10.1093/infdis/141.6.738. [DOI] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Marshall J. H., Skurray R. A. Plasmid-mediated antibiotic resistance in methicillin-resistant Staphylococcus aureus. Med J Aust. 1982 May 29;1(11):468–469. doi: 10.5694/j.1326-5377.1982.tb132418.x. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193(3):554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo J., Noble W. C., Weissmann A., Dyke K. G. Gentamicin-resistant staphylococci: genetics of an outbreak in a dermatology department. J Hyg (Lond) 1983 Aug;91(1):7–16. doi: 10.1017/s0022172400059970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., de Azavedo J. C., Kennedy S., Foster T. J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog. 1986 Apr;1(2):125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shannon K., Phillips I. Mechanisms of resistance to aminoglycosides in clinical isolates. J Antimicrob Chemother. 1982 Feb;9(2):91–102. doi: 10.1093/jac/9.2.91. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Greed L. C., Grubb W. B. Analysis of plasmids mediating gentamicin resistance in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1984 Apr;13(4):347–352. doi: 10.1093/jac/13.4.347. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Greed L. C., Grubb W. B. Transposition of gentamicin resistance to staphylococcal plasmids encoding resistance to cationic agents. J Antimicrob Chemother. 1984 Aug;14(2):115–124. doi: 10.1093/jac/14.2.115. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Grubb W. B., Ashdown N. Gentamicin resistance in methicillin-resistant Staphylococcus aureus. Pathology. 1983 Apr;15(2):169–174. doi: 10.3109/00313028309084707. [DOI] [PubMed] [Google Scholar]
- Vogel L., Nathan C., Sweeney H. M., Kabins S. A., Cohen S. Infections due to gentamicin-resistant Staphylococcus aureus strain in a nursery for neonatal infants. Antimicrob Agents Chemother. 1978 Mar;13(3):466–472. doi: 10.1128/aac.13.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. R., Skinner S. E., Shaw W. V. Analysis of two chloramphenicol resistance plasmids from Staphylococcus aureus: insertional inactivation of Cm resistance, mapping of restriction sites, and construction of cloning vehicles. Plasmid. 1981 May;5(3):245–258. doi: 10.1016/0147-619x(81)90002-0. [DOI] [PubMed] [Google Scholar]