Abstract
BACKGROUND/AIMS—The autonomic pupillary changes in type I and II diabetic patients without clinical evidence of diabetic autonomic neuropathy (DAN) were compared with age matched controls. The relation between pupillary and cardiovascular autonomic function was assessed in the diabetic patients. METHODS—A case-control study was performed with diabetics grouped according to type and duration of diabetes. Static infrared pupillography was used to compare mean dark adapted pupil size and mean percentage changes in pupil size with pilocarpine 0.1% and cocaine 4% in the diabetic and control groups. All diabetic patients underwent cardiovascular autonomic function assessment using the Valsalva ratio, the 30:15 ratio, and testing for orthostatic hypotension. RESULTS—In total, 72 type I and 69 type II diabetic patients were compared with 120 controls. Mean dark adapted pupil size was significantly smaller in diabetic groups than controls. Except for type I diabetics with disease for less than 5 years, all patient groups had significantly greater mean percentage constriction in pupil size in response to dilute pilocarpine than controls. There was no significant difference between the mean percentage dilatation in response to cocaine 4% in diabetics and controls. A high proportion of patients had normal cardiovascular autonomic function particularly when this was assessed with the Valsalva ratio. CONCLUSIONS—Denervation hypersensitivity to dilute pilocarpine is a result of damage to the pupillary parasympathetic supply of diabetic patients. This occurs before the pupillary sympathetic pathway is affected, it can be detected early in the disease, and it may be a possible explanation for the small pupil size seen in diabetic patients. Pupillary autonomic dysfunction occurs before cardiovascular autonomic changes and detection of pupil denervation hypersensitivity to dilute pilocarpine is an inexpensive way to detect early DAN.
Full Text
The Full Text of this article is available as a PDF (120.1 KB).
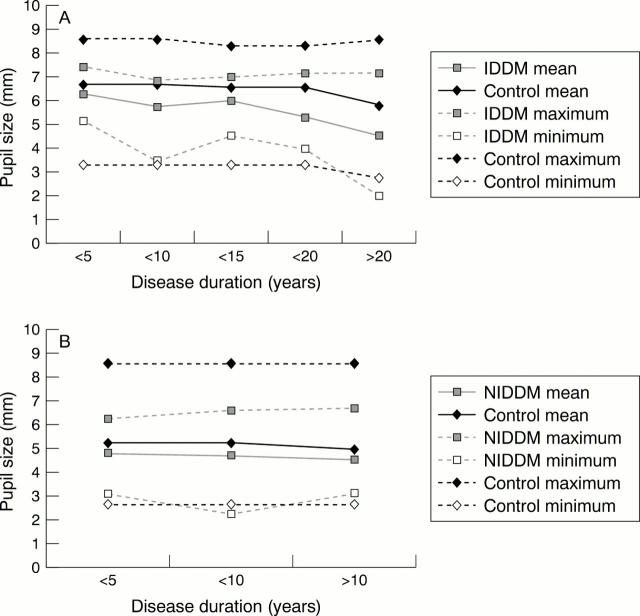
Figure 1 .
Pupillary autonomic denervation with increasing duration of diabetes mellitus. (A) Dark adapted pupil size, IDDM versus controls. (B) Dark adapted pupil size, NIDDM versus controls.
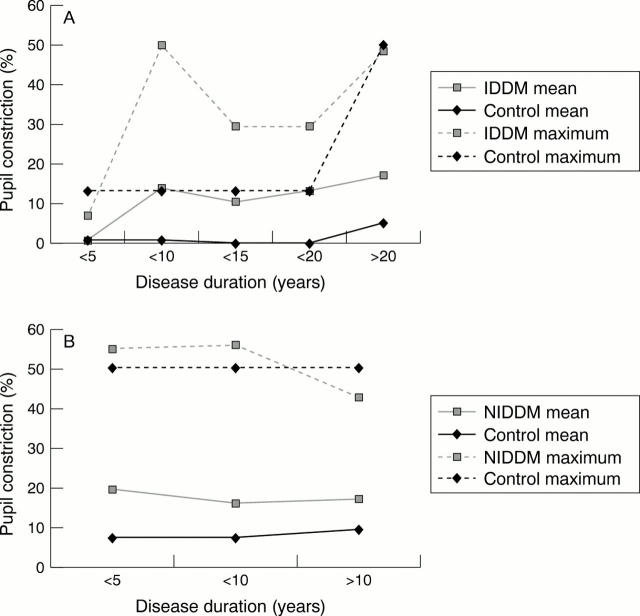
Figure 2 .
Pupillary autonomic denervation with increasing duration of diabetes mellitus. (A) Percentage pupillary constriction with pilocarpine 0.1%, IDDM versus controls. (B) Percentage pupillary constriction with pilocarpine 0.1%, NIDDM versus controls. Note: All minimum values were zero in both patients and controls in (A) and (B)
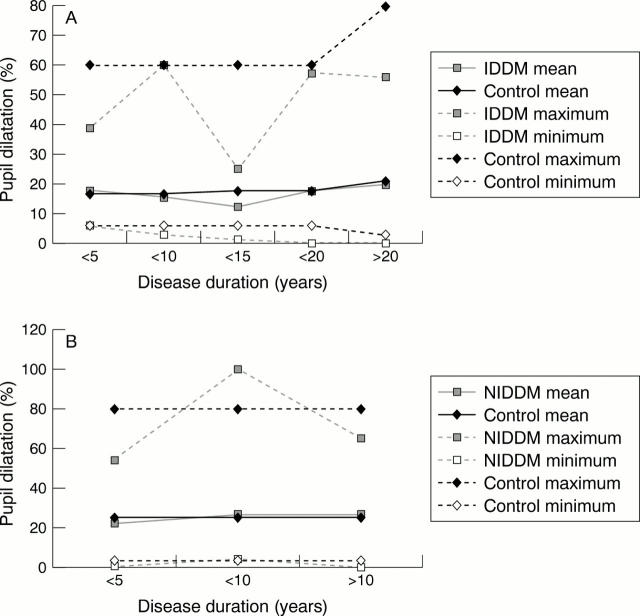
Figure 3 .
Pupillary autonomic denervation with increasing duration of diabetes mellitus. (A) Percentage pupillary dilatation with cocaine 4%, IDDM versus controls. (B) Percentage pupillary dilatation with cocaine 4%, NIDDM versus controls.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron S. A., Rogovski Z., Kanter Y., Hemli Y. Parasympathetic autonomic neuropathy in diabetes mellitus: the heart is denervated more often than the pupil. Electromyogr Clin Neurophysiol. 1994 Dec;34(8):467–469. [PubMed] [Google Scholar]
- Bourgon P., Pilley F. J., Thompson H. S. Cholinergic supersensitivity of the iris sphincter in Adie's tonic pupil. Am J Ophthalmol. 1978 Mar;85(3):373–377. doi: 10.1016/s0002-9394(14)77733-5. [DOI] [PubMed] [Google Scholar]
- Clarke C. F., Piesowicz A. T., Spathis G. S. Pupillary size in children and adolescents with type 1 diabetes. Diabet Med. 1989 Dec;6(9):780–783. doi: 10.1111/j.1464-5491.1989.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Ewing D. J., Campbell I. W., Burt A. A., Clarke B. F. Vascular reflexes in diabetic autonomic neuropathy. Lancet. 1973 Dec 15;2(7842):1354–1356. doi: 10.1016/s0140-6736(73)93323-0. [DOI] [PubMed] [Google Scholar]
- Ewing D. J., Campbell I. W., Clarke B. F. Assessment of cardiovascular effects in diabetic autonomic neuropathy and prognostic implications. Ann Intern Med. 1980 Feb;92(2 Pt 2):308–311. doi: 10.7326/0003-4819-92-2-308. [DOI] [PubMed] [Google Scholar]
- Ewing D. J., Campbell I. W., Murray A., Neilson J. M., Clarke B. F. Immediate heart-rate response to standing: simple test for autonomic neuropathy in diabetes. Br Med J. 1978 Jan 21;1(6106):145–147. doi: 10.1136/bmj.1.6106.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hreidarsson A. B. Pupil size in insulin-dependent diabetes. Relationship to duration, metabolic control, and long-term manifestations. Diabetes. 1982 May;31(5 Pt 1):442–448. doi: 10.2337/diab.31.5.442. [DOI] [PubMed] [Google Scholar]
- Jacobson D. M. Pupillary responses to dilute pilocarpine in preganglionic 3rd nerve disorders. Neurology. 1990 May;40(5):804–808. doi: 10.1212/wnl.40.5.804. [DOI] [PubMed] [Google Scholar]
- Jacobson D. M., Vierkant R. A. Comparison of cholinergic supersensitivity in third nerve palsy and Adie's syndrome. J Neuroophthalmol. 1998 Sep;18(3):171–175. [PubMed] [Google Scholar]
- Lanting P., Bos J. E., Aartsen J., Schuman L., Reichert-Thoen J., Heimans J. J. Assessment of pupillary light reflex latency and darkness adapted pupil size in control subjects and in diabetic patients with and without cardiovascular autonomic neuropathy. J Neurol Neurosurg Psychiatry. 1990 Oct;53(10):912–914. doi: 10.1136/jnnp.53.10.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting P., Heimans J. J., Reulen J. P., Nauta J., van der Veen E. A. Pupillary light reflex and quantitative sensory and motor neural function tests in diabetic patients. J Neurol. 1988 Mar;235(4):245–247. doi: 10.1007/BF00314357. [DOI] [PubMed] [Google Scholar]
- Loewenfeld I. E., Thompson H. S. The tonic pupil: a re-evaluation. Am J Ophthalmol. 1967 Jan;63(1):46–87. doi: 10.1016/0002-9394(67)90579-x. [DOI] [PubMed] [Google Scholar]
- Low P. A. Diabetic autonomic neuropathy. Semin Neurol. 1996 Jun;16(2):143–151. doi: 10.1055/s-2008-1040970. [DOI] [PubMed] [Google Scholar]
- Pfeifer M. A., Cook D., Brodsky J., Tice D., Parrish D., Reenan A., Halter J. B., Porte D., Jr Quantitative evaluation of sympathetic and parasympathetic control of iris function. Diabetes Care. 1982 Sep-Oct;5(5):518–528. doi: 10.2337/diacare.5.5.518. [DOI] [PubMed] [Google Scholar]
- Purcell J. J., Jr, Krachmer J. H., Thompson H. S. Corneal sensation in Adie's syndrome. Am J Ophthalmol. 1977 Oct;84(4):496–500. doi: 10.1016/0002-9394(77)90440-8. [DOI] [PubMed] [Google Scholar]
- Schwingshandl J., Simpson J. M., Donaghue K., Bonney M. A., Howard N. J., Silink M. Pupillary abnormalities in type I diabetes occurring during adolescence. Comparisons with cardiovascular reflexes. Diabetes Care. 1993 Apr;16(4):630–633. doi: 10.2337/diacare.16.4.630. [DOI] [PubMed] [Google Scholar]
- Sharma S., Hoskin-Mott A., Benstead T., Maxner C. Correlation of the pilo-pupil ratio average, a new test for autonomic denervation, to the severity of diabetic retinopathy. Can J Ophthalmol. 1997 Apr;32(3):170–174. [PubMed] [Google Scholar]
- Smith S. A., Dewhirst R. R. A simple diagnostic test for pupillary abnormality in diabetic autonomic neuropathy. Diabet Med. 1986 Jan;3(1):38–41. doi: 10.1111/j.1464-5491.1986.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Smith S. E. Evidence for a neuropathic aetiology in the small pupil of diabetes mellitus. Br J Ophthalmol. 1983 Feb;67(2):89–93. doi: 10.1136/bjo.67.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. A., Smith S. E. Reduced pupillary light reflexes in diabetic autonomic neuropathy. Diabetologia. 1983 May;24(5):330–332. doi: 10.1007/BF00251818. [DOI] [PubMed] [Google Scholar]
- Smith S. E., Smith S. A., Brown P. M., Fox C., Sönksen P. H. Pupillary signs in diabetic autonomic neuropathy. Br Med J. 1978 Sep 30;2(6142):924–927. doi: 10.1136/bmj.2.6142.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub R. H., Jeron A., Kerp L. The pupillary light reflex. 2. Prevalence of pupillary autonomic neuropathy in diabetics using age-dependent and age-independent pupillary parameters. Ophthalmologica. 1992;204(3):143–148. doi: 10.1159/000310283. [DOI] [PubMed] [Google Scholar]
- Straub R. H., Thies U., Jeron A., Palitzsch K. D., Schölmerich J. Valid parameters for investigation of the pupillary light reflex in normal and diabetic subjects shown by factor analysis and partial correlation. Diabetologia. 1994 Apr;37(4):414–419. doi: 10.1007/BF00408480. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Utsumi T., Miyashita Y., Moriya S., Suzuki M., Maetani S. [A re-evaluation of pupillary sensitivity to autonomics in diabetic patients]. Nippon Ganka Gakkai Zasshi. 1990 Apr;94(4):413–417. [PubMed] [Google Scholar]
- Utsumi T. Corneal permeability in patients with tonic pupil. A reevaluation of its cholinergic supersensitivity. J Clin Neuroophthalmol. 1990 Mar;10(1):52–55. [PubMed] [Google Scholar]
- Wirtschafter J. D., Volk C. R., Sawchuk R. J. Transaqueous diffusion of acetylcholine to denervated iris sphincter muscle: a mechanism for the tonic pupil syndrome (Adie syndrome). Ann Neurol. 1978 Jul;4(1):1–5. doi: 10.1002/ana.410040102. [DOI] [PubMed] [Google Scholar]