Abstract
AIM—To evaluate the cell proliferation activity in posterior uveal melanomas after Ru-106 brachytherapy. METHODS—Eyes containing choroidal or ciliary body melanoma from seven ocular oncology centres, which were enucleated after first being treated by Ru-106 brachytherapy and which had enough melanoma tissue to enable histological assessment, were included. The 57 eligible specimens were divided into a group of 44 eyes that were enucleated because of tumour regrowth, and a non-recurrent group of 13 eyes that were enucleated because of complications such as neovascular glaucoma. 46 non-irradiated eyes harbouring uveal melanoma served as a control group. All specimens underwent routine processing. They were cut into 5 µm sections, and were stained with two main cell proliferation markers: PC-10 for PCNA and MIB-1 for Ki-67. The stained sections were assessed, and the cells that were positive in the immunostaining were counted in each section. The results were evaluated by various statistical methods. RESULTS—The PC-10 score showed a statistically significant difference across the three groups (p = 0.002). The control group showed the highest PC-10 score (median 31.0 PCC/HPF) followed by the tumour regrowth group (median 4.9 PCC/HPF). The lowest PC-10 scores were found in the non-recurrent tumours (median 0.05 PCC/HPF). The MIB-1 score in the control group (median 5.77 PCC/HPF) was similar to the regrowth group (median 5.4 PCC/HPF). In contrast, the MIB-1 score in the non-recurrent tumours was statistically significantly lower (median 0.42 PCC/HPF). The PC-10 and MIB-1 scores were similar in tumours composed of either spindle cells or epithelioid cells in all groups. CONCLUSIONS—The non-recurrent melanomas demonstrate significantly lower cellular proliferation activity than melanomas that showed regrowth or that were not irradiated at all. In our hands, PCNA gave more meaningful information than Ki-67. Our findings strongly support the need for treating regrowing posterior uveal melanoma either by enucleation or re-treatment by brachytherapy. On the other hand, also in the non-recurrent uveal melanomas there are viable cells with potential for proliferation, although fewer in number, with unknown capacity for metastatic spread. Therefore, the irradiated tumours should be followed for many years, probably for life.
Full Text
The Full Text of this article is available as a PDF (112.7 KB).
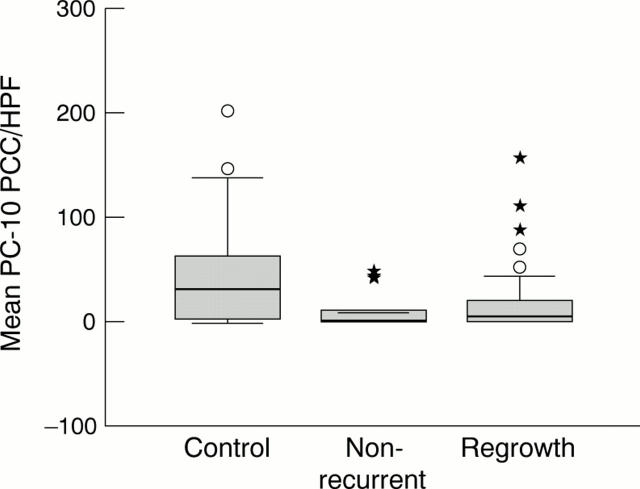
Figure 1 .
Box plot analyses of the proliferative activity of melanoma cells in the control, non-recurrent, and regrowth groups as estimated by PC-10 immunostaining. The thick horizontal lines show the median of the distribution of PC-10 positive cell count (PCC) per HPF, the boxes represent the interquartile range (IQR), and individual circles and asterisks represent outliers. A circle is more than 1.5 times IQR above the upper quartile, and an asterisk is more than three times IQR above the upper quartile.
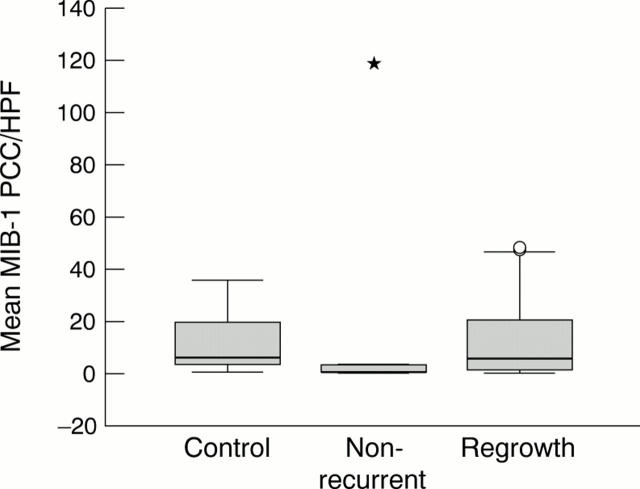
Figure 2 .
Box plot analyses of the proliferative activity of melanoma cells in the control, non-recurrent, and regrowth groups as estimated by MIB-1 immunostaining. The thick horizontal lines show the median of the distribution of MIB-1 positive cell count (PCC) per HPF, the boxes represent the interquartile range (IQR), and individual circles and asterisks represent outliers. A circle is more than 1.5 times IQR above the upper quartile, and an asterisk is more than three times IQR above the upper quartile.
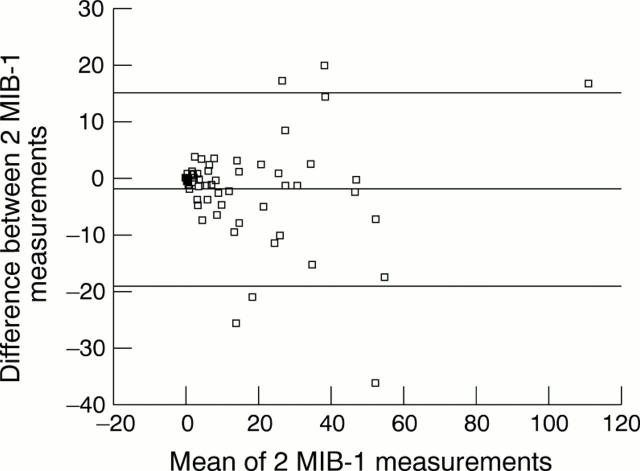
Figure 3 .
Interobserver agreement on the MIB-1 score
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Char D. H., Crawford J. B., Kaleta-Michaels S., Howes E. L., Jr, Lovato A. A., Quivey J. M. Analysis of radiation failure after uveal melanoma brachytherapy. Am J Ophthalmol. 1989 Dec 15;108(6):712–716. doi: 10.1016/0002-9394(89)90867-2. [DOI] [PubMed] [Google Scholar]
- Char D. H., Saunders W., Castro J. R., Quivey J. M., Irvine A. R., Stone R. D., Crawford J. B., Barricks M., Lonn L. I., Hilton G. F. Helium ion therapy for choroidal melanoma. Ophthalmology. 1983 Oct;90(10):1219–1225. doi: 10.1016/s0161-6420(83)34405-5. [DOI] [PubMed] [Google Scholar]
- Chiquet C., Grange J. D., Ayzac L., Chauvel P., Patricot L. M., Devouassoux-Shisheboran M. Effects of proton beam irradiation on uveal melanomas: a comparative study of Ki-67 expression in irradiated versus non-irradiated melanomas. Br J Ophthalmol. 2000 Jan;84(1):98–102. doi: 10.1136/bjo.84.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. B., Char D. H. Histopathology of uveal melanomas treated with charged particle radiation. Ophthalmology. 1987 Jun;94(6):639–643. doi: 10.1016/s0161-6420(87)33399-8. [DOI] [PubMed] [Google Scholar]
- Gragoudas E. S., Goitein M., Verhey L., Munzenreider J., Suit H. D., Koehler A. Proton beam irradiation. An alternative to enucleation for intraocular melanomas. Ophthalmology. 1980 Jun;87(6):571–581. doi: 10.1016/s0161-6420(80)35212-3. [DOI] [PubMed] [Google Scholar]
- Lommatzsch P. K. Experiences in the treatment of malignant melanoma of the choroid with 106Ru-106Rh beta-ray applicators. Trans Ophthalmol Soc U K. 1973;93(0):119–132. [PubMed] [Google Scholar]
- Lommatzsch P. K. Results after beta-irradiation (106Ru/106Rh) of choroidal melanomas: 20 years' experience. Br J Ophthalmol. 1986 Nov;70(11):844–851. doi: 10.1136/bjo.70.11.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch P. K., Werschnik C., Schuster E. Long-term follow-up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 2000 Feb;238(2):129–137. doi: 10.1007/pl00007880. [DOI] [PubMed] [Google Scholar]
- MacFaul P. A., Morgan G. Histopathological changes in malignant melanomas of the choroid after cobalt plaque therapy. Br J Ophthalmol. 1977 Mar;61(3):221–228. doi: 10.1136/bjo.61.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer E., Bornfeld N., Foerster M., Schilling H., Wessing A. Histopathologic findings in eyes treated with a ruthenium plaque for uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 1992;230(4):391–396. doi: 10.1007/BF00165952. [DOI] [PubMed] [Google Scholar]
- Mueller A. J., Talies S., Schaller U. C., Horstmann G., Wowra B., Kampik A. Stereotactic radiosurgery of large uveal melanomas with the gamma-knife. Ophthalmology. 2000 Jul;107(7):1381–1388. doi: 10.1016/s0161-6420(00)00150-0. [DOI] [PubMed] [Google Scholar]
- Ossoinig K. C. Standardized echography: basic principles, clinical applications, and results. Int Ophthalmol Clin. 1979 Winter;19(4):127–210. [PubMed] [Google Scholar]
- Pe'er J., Gnessin H., Shargal Y., Livni N. PC-10 immunostaining of proliferating cell nuclear antigen in posterior uveal melanoma. Enucleation versus enucleation postirradiation groups. Ophthalmology. 1994 Jan;101(1):56–62. doi: 10.1016/s0161-6420(94)38024-9. [DOI] [PubMed] [Google Scholar]
- Petrovich Z., McDonnell J. M., Palmer D., Langholz B. M., Liggett P. E. Histopathologic changes following irradiation for uveal tract melanoma. Am J Clin Oncol. 1994 Aug;17(4):298–306. doi: 10.1097/00000421-199408000-00004. [DOI] [PubMed] [Google Scholar]
- STALLARD H. B. Malignant melanoma of the choroid treated with radioactive applicators. Trans Ophthalmol Soc U K. 1959;79:373–392. [PubMed] [Google Scholar]
- Saornil M. A., Egan K. M., Gragoudas E. S., Seddon J. M., Walsh S. M., Albert D. M. Histopathology of proton beam-irradiated vs enucleated uveal melanomas. Arch Ophthalmol. 1992 Aug;110(8):1112–1118. doi: 10.1001/archopht.1992.01080200092031. [DOI] [PubMed] [Google Scholar]
- Saornil M. A., Fisher M. R., Campbell R. J., Robertson D. M., Earle J. D., Eagle R. C., Jr, Shields J. A., Shields C. L., Chandra S. R., Albert D. M. Histopathologic study of eyes after iodine I 125 episcleral plaque irradiation for uveal melanoma. Arch Ophthalmol. 1997 Nov;115(11):1395–1400. doi: 10.1001/archopht.1997.01100160565006. [DOI] [PubMed] [Google Scholar]
- Schilling H., Bornfeld N., Friedrichs W., Pauleikhoff D., Sauerwein W., Wessing A. Histopathologic findings in large uveal melanomas after brachytherapy with iodine 125 ophthalmic plaques. Ger J Ophthalmol. 1994 Aug;3(4-5):232–238. [PubMed] [Google Scholar]
- Schilling H., Sehu K. W., Lee W. R. A histologic study (including DNA quantification and Ki-67 labeling index) in uveal melanomas after brachytherapy with ruthenium plaques. Invest Ophthalmol Vis Sci. 1997 Sep;38(10):2081–2092. [PubMed] [Google Scholar]
- Sealy R., le Roux P. L., Rapley F., Hering E., Shackleton D., Sevel D. The treatment of ophthalmic tumours with low-energy sources. Br J Radiol. 1976 Jun;49(582):551–554. doi: 10.1259/0007-1285-49-582-551. [DOI] [PubMed] [Google Scholar]
- Seregard S., Lundell G., Lax I., af Trampe E., Kock E. Tumour cell proliferation after failed ruthenium plaque radiotherapy for posterior uveal melanoma. Acta Ophthalmol Scand. 1997 Apr;75(2):148–154. doi: 10.1111/j.1600-0420.1997.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Seregard S., Oskarsson M., Spångberg B. PC-10 as a predictor of prognosis after antigen retrieval in posterior uveal melanoma. Invest Ophthalmol Vis Sci. 1996 Jun;37(7):1451–1458. [PubMed] [Google Scholar]
- Shapiro B. E., Felberg N. T., Donoso L. A., Augsburger J. J., Shields J. A., Gamel J. Flow cytometry of uveal melanomas. Cancer Biochem Biophys. 1986 Jul;8(3):235–238. [PubMed] [Google Scholar]
- Shields C. L., Shields J. A., Karlsson U., Menduke H., Brady L. W. Enucleation after plaque radiotherapy for posterior uveal melanoma. Histopathologic findings. Ophthalmology. 1990 Dec;97(12):1665–1670. doi: 10.1016/s0161-6420(90)32363-1. [DOI] [PubMed] [Google Scholar]
- Zehetmayer M., Kitz K., Menapace R., Ertl A., Heinzl H., Ruhswurm I., Georgopoulos M., Dieckmann K., Pötter R. Local tumor control and morbidity after one to three fractions of stereotactic external beam irradiation for uveal melanoma. Radiother Oncol. 2000 May;55(2):135–144. doi: 10.1016/s0167-8140(00)00164-x. [DOI] [PubMed] [Google Scholar]