Abstract
BACKGROUND/AIM—It was previously reported that collagenous extracellular matrix (ECM) in human capsular opacification contained isoforms of transforming growth factor β (TGFβ). In the present study, the authors performed immunohistochemistry to examine whether ECM in human capsular opacification and in cultures of bovine lens epithelial cells (LECs) contained latent TGFβ binding protein-1 (LTBP-1), TGFβ1 latency associated peptide (β1-LAP), and fibrillin-1, a suspected ligand of LTBP-1 as well as a component of the extracellular microfibrillar apparatus. The aim of the study was to further clarify the mechanism of TGFβ1 deposition in ECM of capsular opacification. METHODS—Human capsular opacification specimens and uninjured lens capsules, as well as cultured bovine LECs, were processed for immunohistochemistry using antibodies against LTBP-1, β1-LAP, fibrillin-1, and collagen type I. RESULTS—LTBP-1, β1-LAP, and fibrillin-1 all were localised to the ECM in human capsular opacification. Uninjured lens epithelium stained for β1-LAP, but not for LTBP-1 and fibrillin-1. ECM deposited in confluent LEC cultures stained for LTBP-1, β1-LAP, and fibrillin-1, while cultures with only sparse cellularity were unstained for LTBP-1 or fibrillin-1. CONCLUSIONS—LECs upregulate LTBP-1 and fibrillin-1 during postoperative healing. LTBP-1, β1-LAP, and fibrillin-1 colocalised to the ECM in capsular opacification and in confluent LEC cultures. TGFβ1 is considered to deposit in ECM in the large latent form. ECM secreted by LEC may function as a scavenger or repository of TGFβ.
Full Text
The Full Text of this article is available as a PDF (198.0 KB).
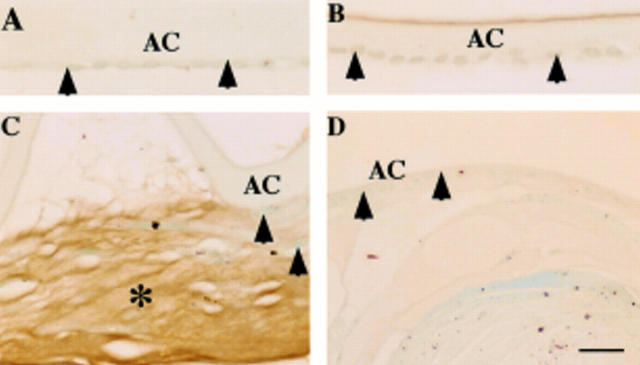
Figure 1 .
Localisation pattern of large transforming growth factor β binding protein-1 (LTBP-1) in human posterior capsule opacification. In (A) (case 1) and (B) (case 8), lens epithelium of an uninjured lens capsule (A, arrowheads) and a capsule 10 days after cataract surgery (B, arrowheads) lack LTBP-1. In (C) (case 17), extracellular matrix accumulation (asterisk) between the anterior lens capsule and the anterior surface of the intraocular lens shows immunoreactivity for LTBP-1. Lens cells are negative for this protein (arrowheads). In (D) (case 19), lens cells (arrowheads) and regenerated lenticular fibres of Sommerring's ring unstained for LTBP-1. AC = anterior capsule; IOL = intraocular lens. Indirect immunostaining; bar = 20 µm.
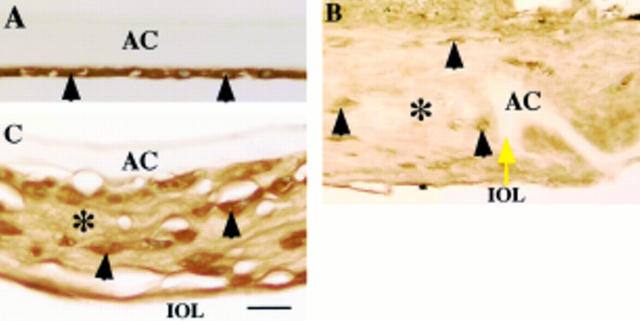
Figure 2 .
Localisation pattern of β1 latency associated peptide (β1-LAP) in human posterior capsule opacification. In (A) (case 1), lens epithelial cells (arrowheads) of an uninjured lens capsule exhibit immunoreactivity for β1-LAP. In (B) (case 25), extracellular matrix accumulation (asterisk) around the edge of the capsulotomy (yellow arrow) shows weak immunoreactivity for β1-LAP, while lens cells (arrowheads) exhibit marked immunoreactivity. In (C) (case 17), extracellular matrix accumulation (asterisk) between the intraocular lens optic and the anterior capsule as well as lens cells (arrowheads) show strong β1-LAP immunoreactivity. AC = anterior capsule; IOL = intraocular lens. Indirect immunostaining; bar = 20 µm.
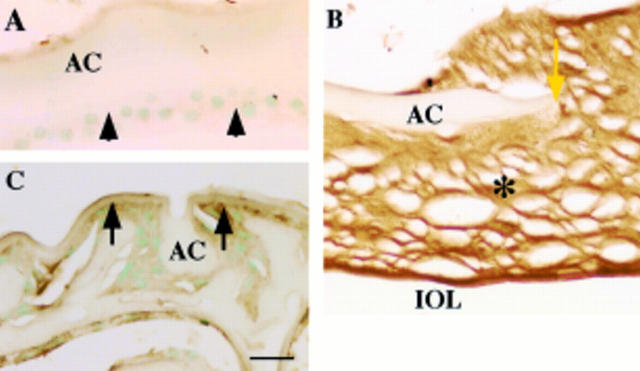
Figure 3 .
Fibrillin-1 as a component of fibrous extracellular matrix in human posterior capsule opacification. In (A) (case 2), uninjured lens epithelium (arrowheads) lacks immunoreactivity for fibrillin-1. In (B) (case 18), extracellular matrix accumulation (asterisk) around the edge of anterior capsulotomy (yellow arrow) shows marked immunoreactivity for fibrillin-1. In (C) (case 17), weak immunoreactivity for fibrillin-1 (arrows) is detected surrounding proliferating lens epithelial cells, which lack immunoreactivity. AC = anterior capsule; IOL = intraocular lens. Indirect immunostaining; bar = 20 µm.
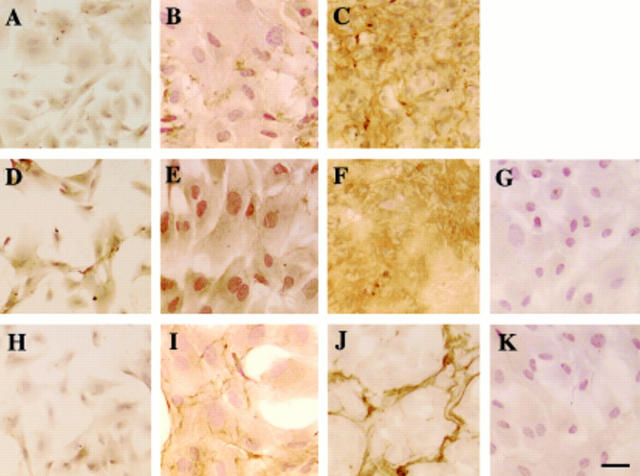
Figure 4 .
Immunolocalisation of LTBP-1, β1-LAP, and fibrillin-1 in the extracellular matrix accumulation in bovine lens epithelial cell cultures. In sparse, preconfluent, cultures at day 3, almost no immunoreactivity for LTBP-1 was detected in LECs (A). In early confluence, a small amount of LTBP-1 was detected extracellularly in a very fine fibrillar pattern (B), while in mature confluent cultures at day 14, immunoreactivity for LTBP-1 appeared to form an organised network throughout the culture (C). Immunoreactivity for β1-LAP was detected in the cells of a sparsely cellular culture (D) and an early confluent culture (E). The mature confluent culture of LECs exhibited extracellular deposition of β1-LAP (F). In sparsely cellular cultures, LECs did not express fibrillin-1 protein (H). In early confluent cultures at day 10, fibrillin-1 was detected in a fine fibrillar pattern in fibroblast ECM; the cells themselves were unstained (I). Mature confluent LEC cultures showed a condensed fibrillin-1 structure (J). No specific immunoreactivity was observed in negative controls with anti-goat IgG (G) or anti-rabbit IgG but no primary antibody (K). LTBP-1 = large transforming growth factor β binding protein-1; β1-LAP = β1-latency associated peptide; LEC = lens epithelial cells. Indirect immunostaining; bar = 50 µm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apple D. J., Solomon K. D., Tetz M. R., Assia E. I., Holland E. Y., Legler U. F., Tsai J. C., Castaneda V. E., Hoggatt J. P., Kostick A. M. Posterior capsule opacification. Surv Ophthalmol. 1992 Sep-Oct;37(2):73–116. doi: 10.1016/0039-6257(92)90073-3. [DOI] [PubMed] [Google Scholar]
- Arteaga C. L., Coffey R. J., Jr, Dugger T. C., McCutchen C. M., Moses H. L., Lyons R. M. Growth stimulation of human breast cancer cells with anti-transforming growth factor beta antibodies: evidence for negative autocrine regulation by transforming growth factor beta. Cell Growth Differ. 1990 Aug;1(8):367–374. [PubMed] [Google Scholar]
- Ashcroft G. S., Yang X., Glick A. B., Weinstein M., Letterio J. L., Mizel D. E., Anzano M., Greenwell-Wild T., Wahl S. M., Deng C. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999 Sep;1(5):260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- Ashworth J. L., Kielty C. M., McLeod D. Fibrillin and the eye. Br J Ophthalmol. 2000 Nov;84(11):1312–1317. doi: 10.1136/bjo.84.11.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins S. W., McCabe M. M., Danielpour D., Streilein J. W. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991 Jul;32(8):2201–2211. [PubMed] [Google Scholar]
- Dallas S. L., Miyazono K., Skerry T. M., Mundy G. R., Bonewald L. F. Dual role for the latent transforming growth factor-beta binding protein in storage of latent TGF-beta in the extracellular matrix and as a structural matrix protein. J Cell Biol. 1995 Oct;131(2):539–549. doi: 10.1083/jcb.131.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Thomson C., de Iongh R. U., Hales A. M., Chamberlain C. G., McAvoy J. W. Differential cataractogenic potency of TGF-beta1, -beta2, and -beta3 and their expression in the postnatal rat eye. Invest Ophthalmol Vis Sci. 1998 Jul;39(8):1399–1409. [PubMed] [Google Scholar]
- Kawashima Y., Saika S., Miyamoto T., Yamanaka O., Okada Y., Tanaka S., Ohnishi Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases of fibrous humans lens capsules with intraocular lenses. Curr Eye Res. 2000 Dec;21(6):962–967. doi: 10.1076/ceyr.21.6.962.6989. [DOI] [PubMed] [Google Scholar]
- Kurosaka D., Kato K., Oshima T., Kurosaka H., Yoshino M., Ogata M. Extracellular matrixes influence alpha-smooth muscle actin expression in cultured porcine lens epithelial cells. Curr Eye Res. 1999 Sep;19(3):260–263. doi: 10.1076/ceyr.19.3.260.5308. [DOI] [PubMed] [Google Scholar]
- Kurosaka D., Nagamoto T. Inhibitory effect of TGF-beta 2 in human aqueous humor on bovine lens epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1994 Aug;35(9):3408–3412. [PubMed] [Google Scholar]
- Levine J. H., Moses H. L., Gold L. I., Nanney L. B. Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am J Pathol. 1993 Aug;143(2):368–380. [PMC free article] [PubMed] [Google Scholar]
- Magro G., Fraggetta F., Travali S., Lanzafame S. Immunohistochemical expression and distribution of alpha2beta1, alpha6beta1, alpha5beta1 integrins and their extracellular ligands, type IV collagen, laminin and fibronectin in palmar fibromatosis. Gen Diagn Pathol. 1997 Dec;143(4):203–208. [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Meacock W. R., Spalton D. J., Stanford M. R. Role of cytokines in the pathogenesis of posterior capsule opacification. Br J Ophthalmol. 2000 Mar;84(3):332–336. doi: 10.1136/bjo.84.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. A., Pelton R. W., Derynck R., Moses H. L. Transforming growth factor-beta. A family of growth regulatory peptides. Ann N Y Acad Sci. 1990;593:208–217. doi: 10.1111/j.1749-6632.1990.tb16113.x. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Miyazono K., Kato M., Takase M., Yamagishi T., Nakamura H. Extracellular fibrillar structure of latent TGF beta binding protein-1: role in TGF beta-dependent endothelial-mesenchymal transformation during endocardial cushion tissue formation in mouse embryonic heart. J Cell Biol. 1997 Jan 13;136(1):193–204. doi: 10.1083/jcb.136.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oharazawa H., Ibaraki N., Lin L. R., Reddy V. N. The effects of extracellular matrix on cell attachment, proliferation and migration in a human lens epithelial cell line. Exp Eye Res. 1999 Dec;69(6):603–610. doi: 10.1006/exer.1999.0723. [DOI] [PubMed] [Google Scholar]
- Raghunath M., Tschödrich-Rotter M., Sasaki T., Meuli M., Chu M. L., Timpl R. Confocal laser scanning analysis of the association of fibulin-2 with fibrillin-1 and fibronectin define different stages of skin regeneration. J Invest Dermatol. 1999 Jan;112(1):97–101. doi: 10.1046/j.1523-1747.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- Raghunath M., Unsöld C., Kubitscheck U., Bruckner-Tuderman L., Peters R., Meuli M. The cutaneous microfibrillar apparatus contains latent transforming growth factor-beta binding protein-1 (LTBP-1) and is a repository for latent TGF-beta1. J Invest Dermatol. 1998 Oct;111(4):559–564. doi: 10.1046/j.1523-1747.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- Rakic J. M., Galand A., Vrensen G. F. Separation of fibres from the capsule enhances mitotic activity of human lens epithelium. Exp Eye Res. 1997 Jan;64(1):67–72. doi: 10.1006/exer.1996.0179. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Osborn M., Schimid E., Weber K., Bloemendal H., Franke W. W. Identification of the cytoskeletal proteins in lens-forming cells, a special epitheloid cell type. Exp Cell Res. 1980 Jun;127(2):309–327. doi: 10.1016/0014-4827(80)90437-1. [DOI] [PubMed] [Google Scholar]
- Saika S., Kawashima Y., Miyamoto T., Okada Y., Tanaka S. I., Ohmi S., Minamide A., Yamanaka O., Ohnishi Y., Ooshima A. Immunolocalization of prolyl 4-hydroxylase subunits, alpha-smooth muscle actin, and extracellular matrix components in human lens capsules with lens implants. Exp Eye Res. 1998 Mar;66(3):283–294. doi: 10.1006/exer.1997.0434. [DOI] [PubMed] [Google Scholar]
- Saika S., Miyamoto T., Kawashima Y., Okada Y., Yamanaka O., Ohnishi Y., Ooshima A. Immunolocalization of TGF-beta1, -beta2, and -beta3, and TGF-beta receptors in human lens capsules with lens implants. Graefes Arch Clin Exp Ophthalmol. 2000 Mar;238(3):283–293. doi: 10.1007/s004170050354. [DOI] [PubMed] [Google Scholar]
- Saika S., Miyamoto T., Okada Y., Yamanaka O., Ohnishi Y., Ooshima A. Transforming growth factor-beta isoform proteins in cell and matrix deposits on intraocular lenses. J Cataract Refract Surg. 2000 May;26(5):709–715. doi: 10.1016/s0886-3350(99)00402-2. [DOI] [PubMed] [Google Scholar]
- Saika S., Ohmi S., Kanagawa R., Tanaka S., Ohnishi Y., Ooshima A., Yamanaka A. Lens epithelial cell outgrowth and matrix formation on intraocular lenses in rabbit eyes. J Cataract Refract Surg. 1996;22 (Suppl 1):835–840. doi: 10.1016/s0886-3350(96)80171-4. [DOI] [PubMed] [Google Scholar]
- Saika S., Okada Y., Miyamoto T., Ohnishi Y., Ooshima A., McAvoy J. W. Smad translocation and growth suppression in lens epithelial cells by endogenous TGFbeta2 during wound repair. Exp Eye Res. 2001 Jun;72(6):679–686. doi: 10.1006/exer.2001.1002. [DOI] [PubMed] [Google Scholar]
- Saika S., Shiraishi A., Liu C. Y., Funderburgh J. L., Kao C. W., Converse R. L., Kao W. W. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000 Jan 28;275(4):2607–2612. doi: 10.1074/jbc.275.4.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S., Yamanaka O., Kawashima Y., Ohkawa K., Ohnishi Y., Ooshima A., Kimura M., Nakano Y., Kao W. W. OPC-15161 suppresses the proliferation of Tenon's capsule fibroblasts and the production of type I collagen and fibronectin stimulated by TGF-beta1 in vitro. Curr Eye Res. 1998 Sep;17(9):933–940. doi: 10.1076/ceyr.17.9.933.5142. [DOI] [PubMed] [Google Scholar]
- Taipale J., Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997 Jan;11(1):51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- Wang X. J., Greenhalgh D. A., Bickenbach J. R., Jiang A., Bundman D. S., Krieg T., Derynck R., Roop D. R. Expression of a dominant-negative type II transforming growth factor beta (TGF-beta) receptor in the epidermis of transgenic mice blocks TGF-beta-mediated growth inhibition. Proc Natl Acad Sci U S A. 1997 Mar 18;94(6):2386–2391. doi: 10.1073/pnas.94.6.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. E., Griffiths C. E., Craven N. M., Shuttleworth C. A., Kielty C. M. Fibrillin-rich microfibrils are reduced in photoaged skin. Distribution at the dermal-epidermal junction. J Invest Dermatol. 1999 May;112(5):782–787. doi: 10.1046/j.1523-1747.1999.00562.x. [DOI] [PubMed] [Google Scholar]