Abstract
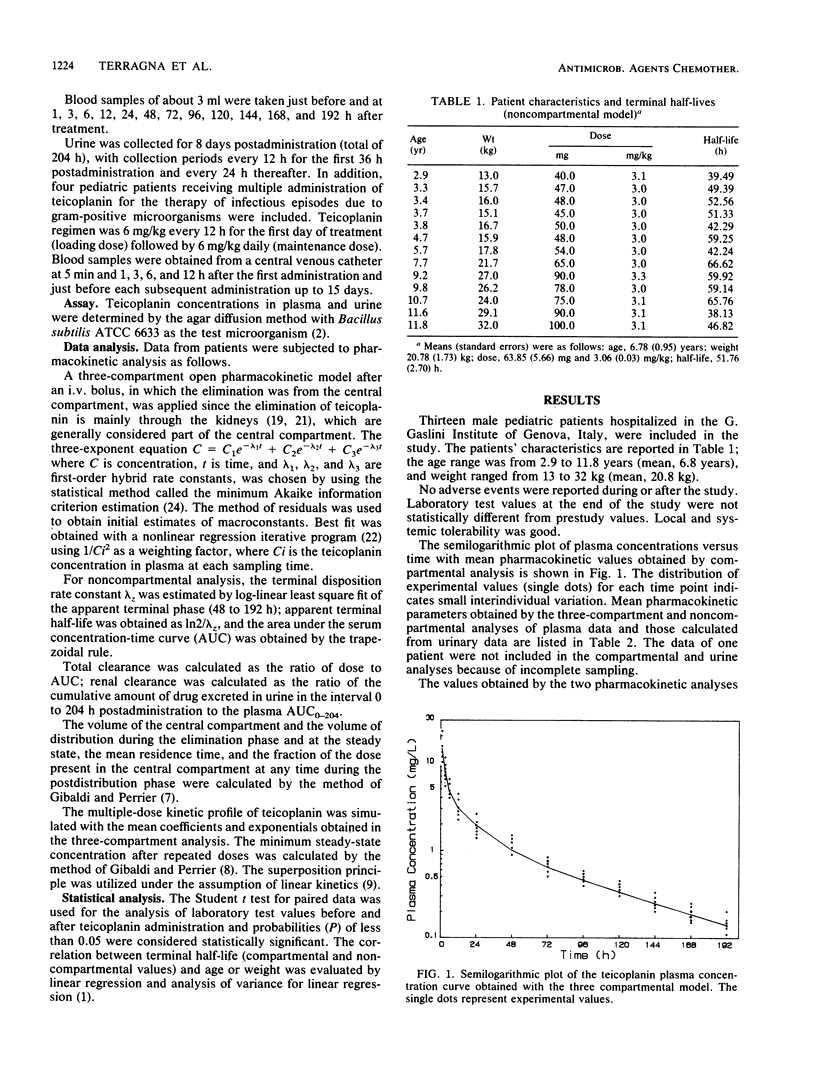
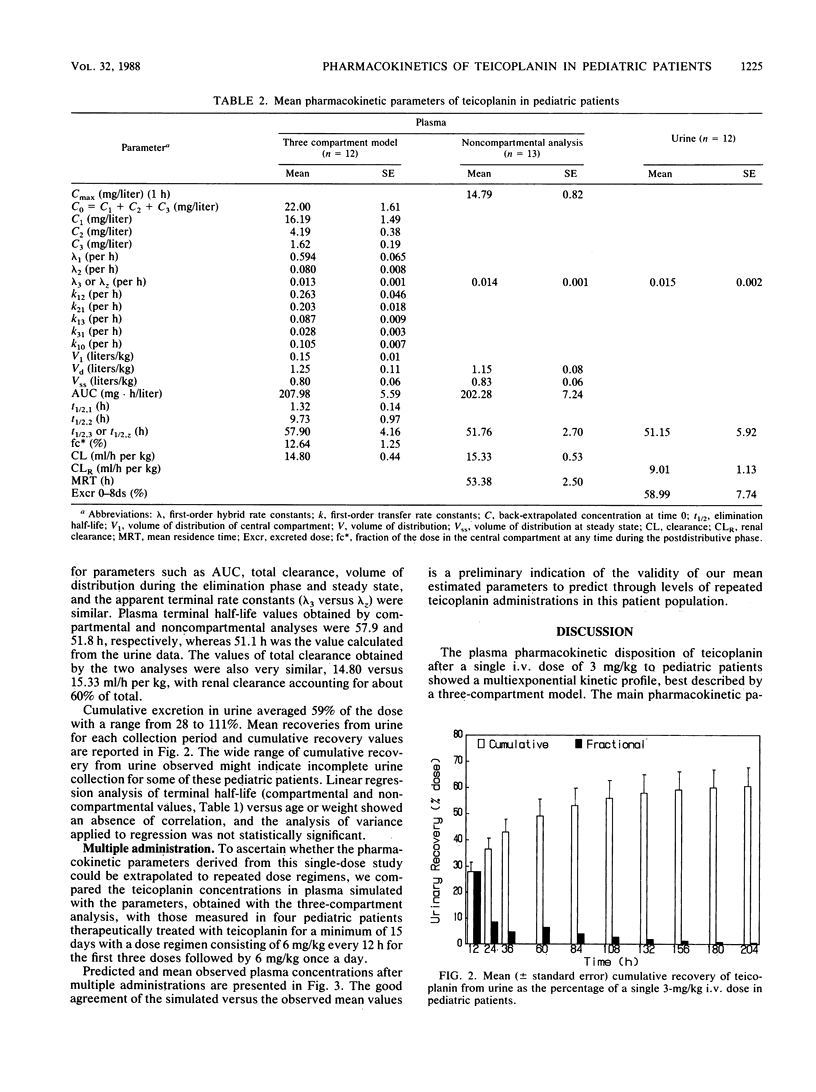
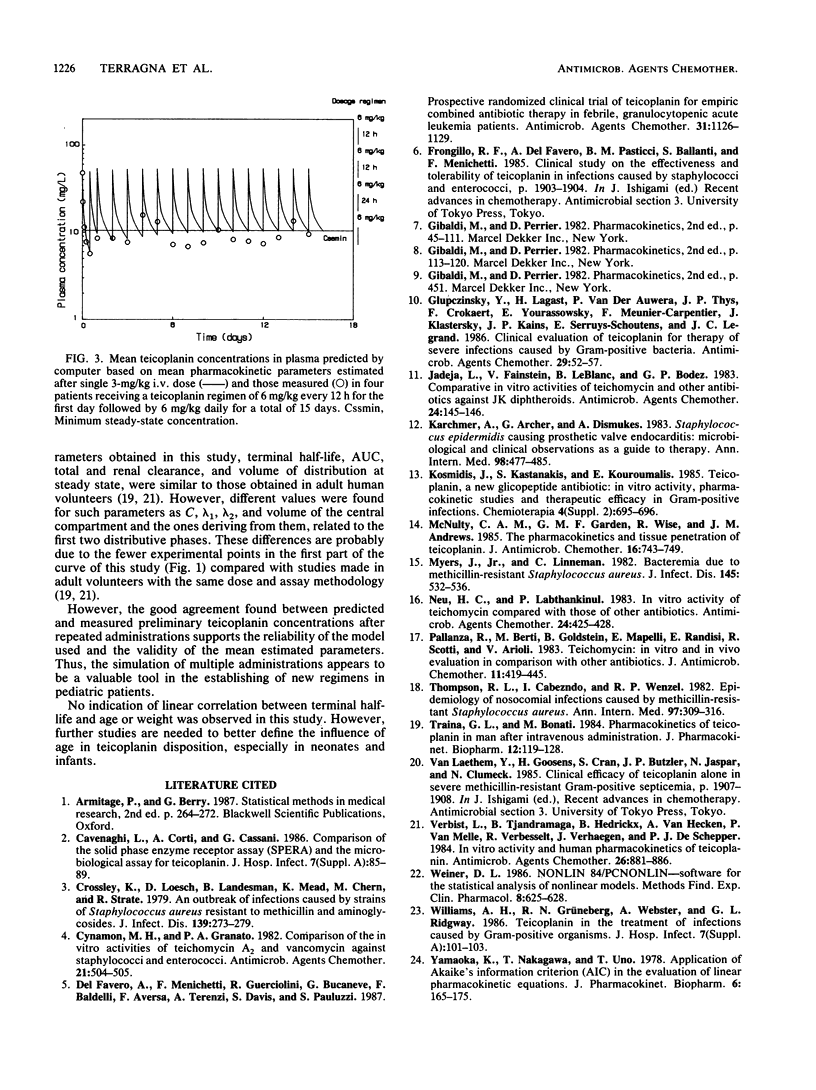
The pharmacokinetics of teicoplanin have been studied in 13 pediatric male patients from 2 to 12 years of age. Patients were given a single 3-mg/kg intravenous dose of teicoplanin for prophylaxis. Blood and urine samples were collected for 8 days after administration, and teicoplanin levels were determined by microbiological assay. Pharmacokinetic parameters were estimated from a three-compartment open pharmacokinetic model and from a noncompartmental analysis. Levels in plasma 1 h after the administration averaged 14.8 mg/liter. The half-lives of the two distribution phases were 1.3 and 9.7 h. The half-life of the terminal phase averaged 57.9 h, with similar estimates obtained from the noncompartmental analysis and from data from urine. The volume of distribution of the central compartment was 0.15 liter/kg, whereas the volume of distribution at steady state and during the elimination phase were 0.80 and 1.25 liters/kg. The total teicoplanin clearance averaged 14.8 ml/h per kg, with renal clearance accounting for about 60% of the total. The average cumulative recovery of teicoplanin in urine over 8 days was 59% of the dose, similar to the value obtained in adult volunteers. There was no significant linear correlation between elimination half-life and age. Preliminary data after repeated administration support the reliability of the model used and the validity of the mean estimated parameters. There were no local or systemic adverse reactions to teicoplanin.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavenaghi L., Corti A., Cassani G. Comparison of the solid phase enzyme receptor assay (SPERA) and the microbiological assay for teicoplanin. J Hosp Infect. 1986 Mar;7 (Suppl A):85–89. doi: 10.1016/0195-6701(86)90012-5. [DOI] [PubMed] [Google Scholar]
- Crossley K., Loesch D., Landesman B., Mead K., Chern M., Strate R. An outbreak of infections caused by strains of Staphylococcus aureus resistant to methicillin and aminoglycosides. I. Clinical studies. J Infect Dis. 1979 Mar;139(3):273–279. doi: 10.1093/infdis/139.3.273. [DOI] [PubMed] [Google Scholar]
- Cynamon M. H., Granato P. A. Comparison of the in vitro activities of teichomycin A2 and vancomycin against staphylococci and enterococci. Antimicrob Agents Chemother. 1982 Mar;21(3):504–505. doi: 10.1128/aac.21.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Favero A., Menichetti F., Guerciolini R., Bucaneve G., Baldelli F., Aversa F., Terenzi A., Davis S., Pauluzzi S. Prospective randomized clinical trial of teicoplanin for empiric combined antibiotic therapy in febrile, granulocytopenic acute leukemia patients. Antimicrob Agents Chemother. 1987 Jul;31(7):1126–1129. doi: 10.1128/aac.31.7.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glupczynski Y., Lagast H., Van der Auwera P., Thys J. P., Crokaert F., Yourassowsky E., Meunier-Carpentier F., Klastersky J., Kains J. P., Serruys-Schoutens E. Clinical evaluation of teicoplanin for therapy of severe infections caused by gram-positive bacteria. Antimicrob Agents Chemother. 1986 Jan;29(1):52–57. doi: 10.1128/aac.29.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadeja L., Fainstein V., LeBlanc B., Bodey G. P. Comparative in vitro activities of teichomycin and other antibiotics against JK diphtheroids. Antimicrob Agents Chemother. 1983 Aug;24(2):145–146. doi: 10.1128/aac.24.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty C. A., Garden G. M., Wise R., Andrews J. M. The pharmacokinetics and tissue penetration of teicoplanin. J Antimicrob Chemother. 1985 Dec;16(6):743–749. doi: 10.1093/jac/16.6.743. [DOI] [PubMed] [Google Scholar]
- Myers J. P., Linnemann C. C., Jr Bacteremia due to methicillin-resistant Staphylococcus aureus. J Infect Dis. 1982 Apr;145(4):532–536. doi: 10.1093/infdis/145.4.532. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity of teichomycin compared with those of other antibiotics. Antimicrob Agents Chemother. 1983 Sep;24(3):425–428. doi: 10.1128/aac.24.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanza R., Berti M., Goldstein B. P., Mapelli E., Randisi E., Scotti R., Arioli V. Teichomycin: in-vitro and in-vivo evaluation in comparison with other antibiotics. J Antimicrob Chemother. 1983 May;11(5):419–425. doi: 10.1093/jac/11.5.419. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Cabezudo I., Wenzel R. P. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):309–317. doi: 10.7326/0003-4819-97-3-309. [DOI] [PubMed] [Google Scholar]
- Traina G. L., Bonati M. Pharmacokinetics of teicoplanin in man after intravenous administration. J Pharmacokinet Biopharm. 1984 Apr;12(2):119–128. doi: 10.1007/BF01059273. [DOI] [PubMed] [Google Scholar]
- Verbist L., Tjandramaga B., Hendrickx B., Van Hecken A., Van Melle P., Verbesselt R., Verhaegen J., De Schepper P. J. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother. 1984 Dec;26(6):881–886. doi: 10.1128/aac.26.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner D. L. NONLIN84/PCNONLIN: software for the statistical analysis of nonlinear models. Methods Find Exp Clin Pharmacol. 1986 Oct;8(10):625–628. [PubMed] [Google Scholar]
- Williams A. H., Grüneberg R. N., Webster A., Ridgway G. L. Teicoplanin in the treatment of infection caused by gram-positive organisms. J Hosp Infect. 1986 Mar;7 (Suppl A):101–103. doi: 10.1016/0195-6701(86)90014-9. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]


