Abstract
AIMS—To evaluate the efficacy of amniotic membrane transplantation (AMT) in persistent corneal epithelial defect with or without stromal thinning and corneal perforation. METHODS—28 patients (28 eyes) with persistent corneal epithelial defect unresponsive to medical treatment were given preserved human amniotic membrane transplants. The patients were divided into three groups: group A, persistent corneal epithelial defect 10 eyes; group B, epithelial defect with stromal thinning 13 eyes; and group C, corneal perforation five eyes. AMT was performed using one layer in group A and multilayers in group B and C. The causes of persistent epithelial defect were neurotrophic keratopathy (24 eyes), limbal deficiency (six eyes), exposure keratopathy (four eyes), and Mooren's ulcer (one eye). RESULTS—Success was noted in 82.1% (23/28 eyes) in all groups, with 80% (8/10 eyes), 84.6% (11/13 eyes), and 80% (4/5 eyes) in groups A, B, and C respectively, with a mean follow up of 10.9 months (1-30 months). The mean epithelialisation time after AMT was 2.1 weeks. The healing times of groups B and C are also significantly shorter than group A (p=0.017 and 0.018, respectively). Corneal stromal thickness was significantly increased in all cases in groups B and C (p=0.006). Those with corneal perforation in group C were completely healed by multilayer AMT. There was no difference in the epithelialisation time between successful cases treated by a single operation (17 eyes) or repeated operation (six eyes). Vision improved in 18.9% (8/28 eyes) and worsened as a result of cataract formation in 2.3% (1/28 eyes). Failure was noted in 17.9% (5/28 eyes), because of corneal infection (two eyes), neurotrophic keratopathy with and without limbal deficiency (two eyes), and intractable corneal perforation (one eye). No patient developed major immediate postoperative complications or graft rejection. CONCLUSION—Amniotic membrane can successfully treat refractory corneal epithelial defect by promoting epithelial healing and thus prevent corneal perforation. It can be used as a treatment for corneal perforation by restoring corneal stromal thickness so that emergency penetrating keratoplasty can be avoided.
Full Text
The Full Text of this article is available as a PDF (287.2 KB).
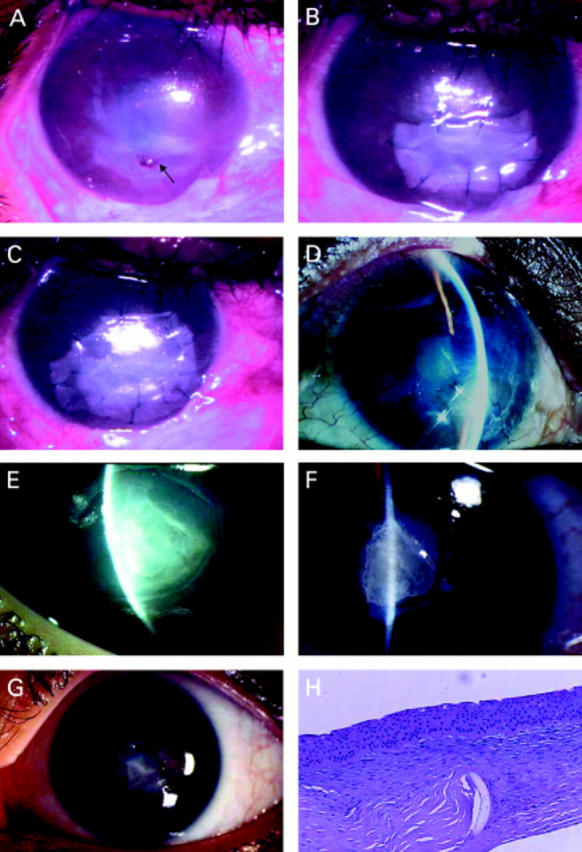
Figure 1 .
Group A. A patient with rhabdomyosarcoma (A) of the eyelid who underwent tumour removal surgery and radiation resulting in upper eyelid defect, with exposure keratopathy and a persistent epithelial defect. Two weeks after AMT and nasal tarsorrhaphy, part of the membrane started to dissolve and the epithelium healed completely (B). A patient with a history of recurrent herpes simplex keratitis (C) and post phacoemulsification, developed a persistent corneal epithelial defect over 1 year. After single layer AMT, the epithelial defect healed completely in 8 weeks but recurred after the amniotic membrane partially dissolved (arrows). A single layer AMT combined with an amniotic membrane patch and tarsorrhaphy were performed (D). One week after AMT, part of the patch dissolved (E). Complete epithelial healing as shown by fluorescein staining (F), which remained stable 5 months thereafter, until optical PKP was performed.
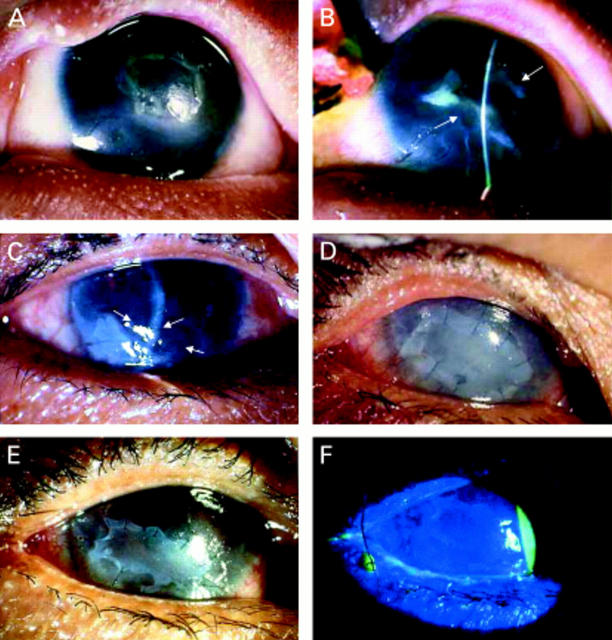
Figure 2 .
Group B. A patient developed a descemetocele from exposure keratopathy after wide excision of a squamous cell carcinoma of the lower eyelid. Appearance at 7 days after multilayer AMT revealed no epithelial defect (A). One month after surgery, the eye showed an increased stromal thickness and a marked reduction of ocular inflammation (B). Surface integrity was maintained up to 9 months before advanced recurrent tumours developed which required exenteration. A patient with Mooren's ulcer developed a corneal perforation (C). Immunosuppressive treatment and glue were used to restore the globe integrity and reduce inflammation (D). Because of the irritative symptoms and glue toxicity, which induced chronic inflammation and prevented tissue healing, the glue was removed and a descemetocele developed which required a multilayer AMT. Complete epithelialisation after 7 postoperative days with a partially dissolved membrane (arrow) (E). Four months after surgery, the eye showed an increased stromal thickness with no inflammation (F).
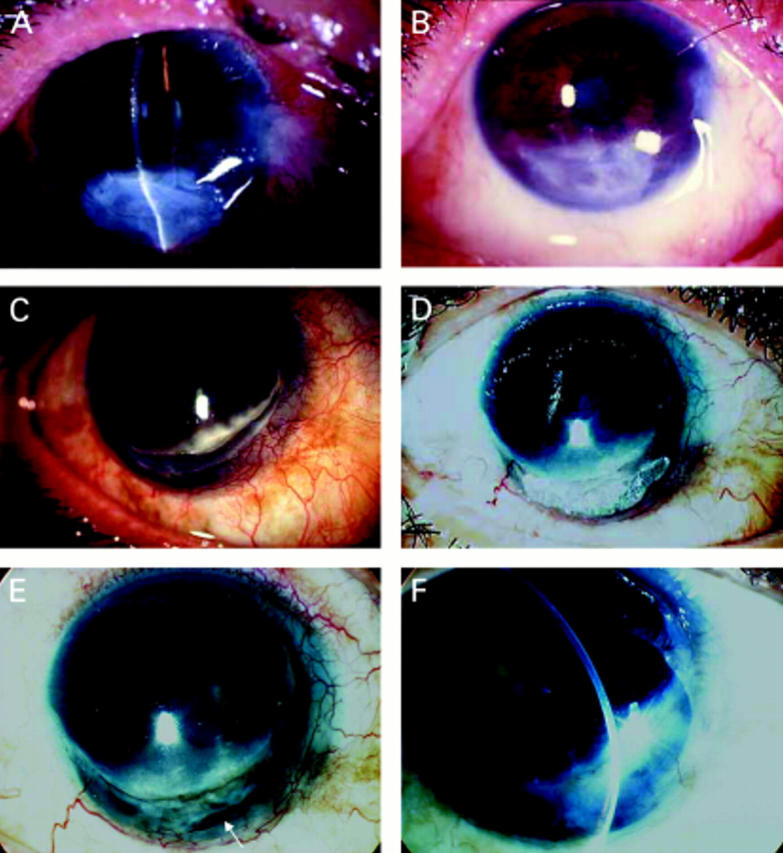
Figure 3 .
Group C. A patient with a history of recurrent herpes simplex virus keratitis developed a corneal perforation (A, arrow). A multilayer AMT was performed (B) with an amniotic patch on top (C). Four months postoperatively, a membrane completely dissolved revealing a fully restored stromal thickness with no epithelial defect (D). The corneal pannus resulted from a previous inflammation induced by herpes. A 3 mm corneal perforation in an eye with herpetic keratitis superimposed by bacterial corneal ulcer was managed by glue and multilayer AMT (E). The membrane dissolved totally after 1 month (F). The exposed glue induced corneal vascularisation and interfered with corneal healing. Removal of glue and repeated multilayer AMT decreased the corneal vascularisation and increased stromal thickness to normal (G). Five months later, PKP was performed which improved the vision from counting fingers to 20/50. A pathological section revealed a normal stromal thickness with abundant keratocytes and a residual part of the membrane in the area of perforation (H).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman M. The pathogenesis of corneal epithelial defects. Acta Ophthalmol Suppl. 1989;192:55–64. doi: 10.1111/j.1755-3768.1989.tb07095.x. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Sympson C. J., Werb Z., Bissell M. J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995 Feb 10;267(5199):891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M., Lamberts D. W., Reid T. W., Nishida T., Murphy C. J. Neurotrophic and anhidrotic keratopathy treated with substance P and insulinlike growth factor 1. Arch Ophthalmol. 1997 Jul;115(7):926–927. doi: 10.1001/archopht.1997.01100160096021. [DOI] [PubMed] [Google Scholar]
- Chen H. J., Pires R. T., Tseng S. C. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000 Aug;84(8):826–833. doi: 10.1136/bjo.84.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua H. S., Azuara-Blanco A. Amniotic membrane transplantation. Br J Ophthalmol. 1999 Jun;83(6):748–752. doi: 10.1136/bjo.83.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S. T. The effect of epidermal growth factor on corneal wound healing: practical considerations for therapeutic use. Refract Corneal Surg. 1991 May-Jun;7(3):232–239. [PubMed] [Google Scholar]
- Fujishima H., Shimazaki J., Shinozaki N., Tsubota K. Trabeculectomy with the use of amniotic membrane for uncontrollable glaucoma. Ophthalmic Surg Lasers. 1998 May;29(5):428–431. [PubMed] [Google Scholar]
- Golubović S., Parunović A. Cyanoacrylate glue in the treatment of corneal ulcerations. Fortschr Ophthalmol. 1990;87(4):378–381. [PubMed] [Google Scholar]
- Hao Y., Ma D. H., Hwang D. G., Kim W. S., Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000 May;19(3):348–352. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- Koizumi N. J., Inatomi T. J., Sotozono C. J., Fullwood N. J., Quantock A. J., Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000 Mar;20(3):173–177. [PubMed] [Google Scholar]
- Kruse F. E., Rohrschneider K., Völcker H. E. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999 Aug;106(8):1504–1511. doi: 10.1016/S0161-6420(99)90444-X. [DOI] [PubMed] [Google Scholar]
- Lee S. B., Li D. Q., Tan D. T., Meller D. C., Tseng S. C. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000 Apr;20(4):325–334. [PubMed] [Google Scholar]
- Lee S. H., Tseng S. C. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997 Mar;123(3):303–312. doi: 10.1016/s0002-9394(14)70125-4. [DOI] [PubMed] [Google Scholar]
- Lugo M., Arentsen J. J. Treatment of neurotrophic ulcers with conjunctival flaps. Am J Ophthalmol. 1987 May 15;103(5):711–712. doi: 10.1016/s0002-9394(14)74337-5. [DOI] [PubMed] [Google Scholar]
- Newsome N. A., Gross J. Prevention by medroxyprogesterone of perforation in the alkali-burned rabbit cornea: inhibition of collagenolytic activity. Invest Ophthalmol Vis Sci. 1977 Jan;16(1):21–31. [PubMed] [Google Scholar]
- Nishida T., Nakagawa S., Manabe R. Clinical evaluation of fibronectin eyedrops on epithelial disorders after herpetic keratitis. Ophthalmology. 1985 Feb;92(2):213–216. doi: 10.1016/s0161-6420(85)34050-2. [DOI] [PubMed] [Google Scholar]
- Pfister R. R. Clinical measures to promote corneal epithelial healing. Acta Ophthalmol Suppl. 1992;(202):73–83. doi: 10.1111/j.1755-3768.1992.tb02172.x. [DOI] [PubMed] [Google Scholar]
- Pfister R. R., Haddox J. L., Dodson R. W., Deshazo W. F. Polymorphonuclear leukocytic inhibition by citrate, other metal chelators, and trifluoperazine. Evidence to support calcium binding protein involvement. Invest Ophthalmol Vis Sci. 1984 Aug;25(8):955–970. [PubMed] [Google Scholar]
- Prabhasawat P., Barton K., Burkett G., Tseng S. C. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997 Jun;104(6):974–985. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- Prabhasawat Pinnita, Kosrirukvongs Panida, Booranapong Wipawee, Vajaradul Yongyudh. Application of Preserved Human Amniotic Membrane for Corneal Surface Reconstruction. Cell Tissue Bank. 2000;1(3):213–222. doi: 10.1023/A:1026542702099. [DOI] [PubMed] [Google Scholar]
- Shimazaki J., Shinozaki N., Tsubota K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br J Ophthalmol. 1998 Mar;82(3):235–240. doi: 10.1136/bjo.82.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki J., Yang H. Y., Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997 Dec;104(12):2068–2076. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Calafat J., Janssen H., Daams H., van der Raaij-Helmer L. M., Falcioni R., Kennel S. J., Aplin J. D., Baker J., Loizidou M. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991 May;113(4):907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. Y., Lin C. P. Combined use of an amniotic membrane and tissue adhesive in treating corneal perforation: a case report. Ophthalmic Surg Lasers. 2000 Mar-Apr;31(2):151–154. [PubMed] [Google Scholar]
- Tseng S. C., Li D. Q., Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999 Jun;179(3):325–335. doi: 10.1002/(SICI)1097-4652(199906)179:3<325::AID-JCP10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Prabhasawat P., Barton K., Gray T., Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998 Apr;116(4):431–441. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Prabhasawat P., Lee S. H. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol. 1997 Dec;124(6):765–774. doi: 10.1016/s0002-9394(14)71693-9. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Tsubota K. Important concepts for treating ocular surface and tear disorders. Am J Ophthalmol. 1997 Dec;124(6):825–835. doi: 10.1016/s0002-9394(14)71700-3. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Satake Y., Ohyama M., Toda I., Takano Y., Ono M., Shinozaki N., Shimazaki J. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol. 1996 Jul;122(1):38–52. doi: 10.1016/s0002-9394(14)71962-2. [DOI] [PubMed] [Google Scholar]
- Welch C., Baum J. Tarsorrhaphy for corneal disease in patients with rheumatoid arthritis. Ophthalmic Surg. 1988 Jan;19(1):31–32. [PubMed] [Google Scholar]