Abstract
AIM—To examine the efficacy of systemic cyclosporin A (CSA) in preventing rejection and graft failure in high risk keratoplasty (PK). METHODS—A retrospective case-control study with 49 patients in both the CSA group and the control group. The patients receiving CSA were at high risk of graft rejection and failure. Controls were identified from surgical audit books and had high risk characteristics. RESULTS—There was no statistical difference in preoperative risk factors and the use of postoperative topical steroids between the two groups. The median follow up in the CSA group was 22 months and 27 months in the control group. One or more rejection episodes occurred in 18 out of 49 (36.7%) cases in the CSA group and 26 out of 49 (53.1%) in the control group. Graft failure from all causes occurred in 16 (32.7%) CSA patients and 18 (36.7%) control patients. Four (8.2%) of the CSA group compared to eight (16.3%) in the control group failed because of rejection. 22 (44.9%) out of 49 patients in the CSA group had side effects. In five (10.2%) patients, CSA was stopped because of the side effects; eight patients had elevated serum urea and creatinine and four developed hypertension. Minor side effects reported include gum hyperplasia, increased sweating, backache, nausea, feeling unwell, oral candidiasis, cramps, and paraesthesia of the extremities. CONCLUSION—These results suggest that the benefit of CSA over conventional therapy in preventing rejection episodes and subsequent graft failure is only moderate and did not reach statistically significant levels in this study. Considering the high frequency of side effects and the cost of CSA, a randomised control trial may be necessary to determine the true value of CSA in high risk penetrating keratoplasty.
Full Text
The Full Text of this article is available as a PDF (206.6 KB).
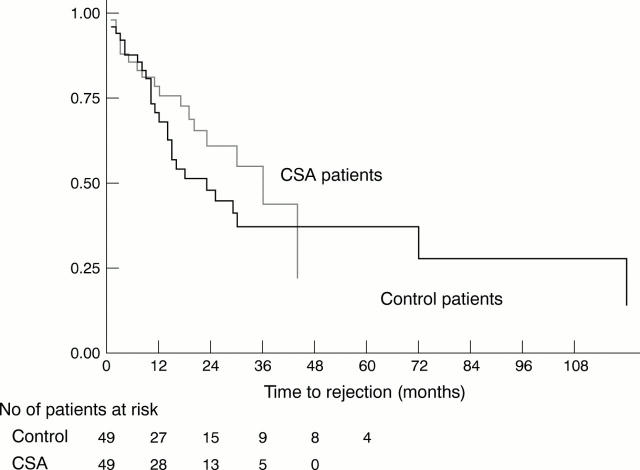
Figure 1 .
Kaplan-Meier survival estimates for rejection. Difference between the CSA and control groups was not significant when compared by log rank and Wilcoxon (Breslow) tests. The number of study patients at risk at various points after time zero are shown below the horizontal axis.
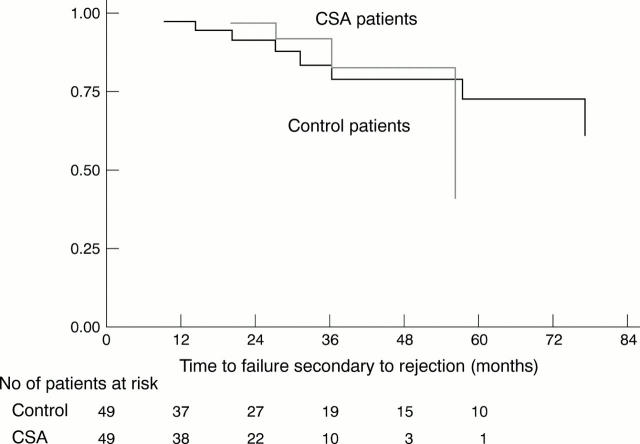
Figure 2 .
Kaplan-Meier survival estimates for failure from rejection. Difference between the CSA and control groups was not significant when compared by log rank and Wilcoxon (Breslow) tests. The number of study patients at risk at various points after time zero are shown below the horizontal axis.
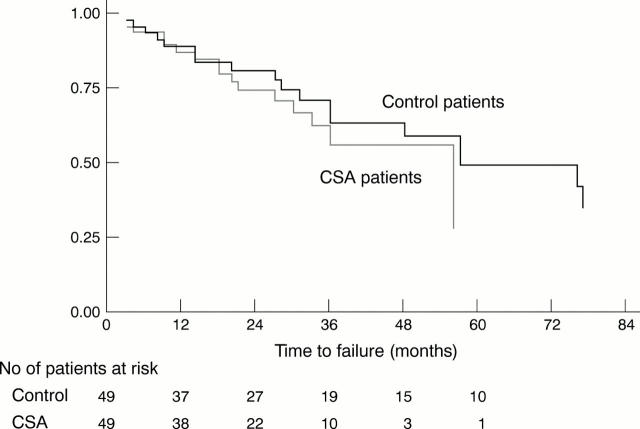
Figure 3 .
Kaplan-Meier survival estimates for failure (all causes). Difference between the CSA and control group was not significant when compared by log rank and Wilcoxon (Breslow) tests. The number of study patients at risk at various points after time zero are shown below the horizontal axis.
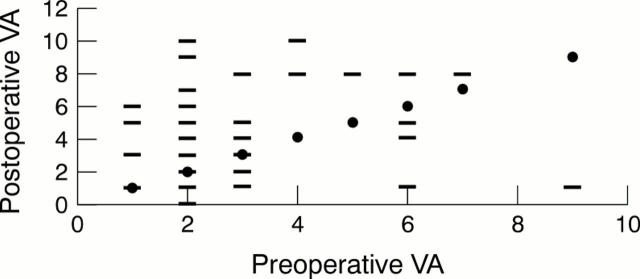
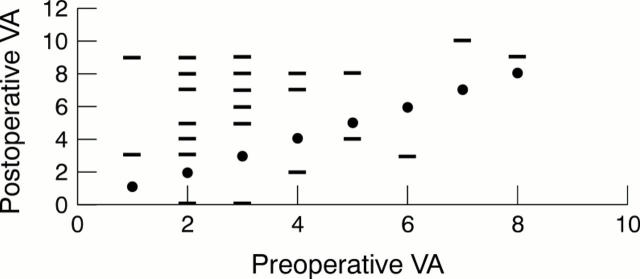
Figure 4 .
Visual acuity (corrected or with pinhole) of control patients, preoperative versus postoperative acuity. VA units: 0 = no perception of light, 1 = perception of light, 2 = hand movement, 3 = counting fingers, 4 = 6/60, 5 = 6/36, 6 = 6/24, 7 = 6/18, 8 = 6/12, 9 = 6/9, 10 = 6/6. Solid circle represents the "no change" line where the postoperative acuity equals preoperative acuity; bar represents the postoperative VA plotted against the preoperative VA. Those above the line of "no change" represent an improvement of VA.
Figure 5 .
Visual acuity (corrected or with pinhole) for CSA patients, preoperative versus postoperative VA. VA units: 0 = no perception of light, 1 = perception of light, 2 = hand movement, 3 = counting fingers, 4 = 6/60, 5 = 6/36, 6 = 6/24, 7 = 6/18, 8 = 6/12, 9 = 6/9, 10 = 6/6. Solid circle represents the "no change" line where the postoperative acuity equals preoperative acuity; bar represents the postoperative VA plotted against the preoperative VA. Those above the line of "no change" represent an improvement of VA.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belin M. W., Bouchard C. S., Frantz S., Chmielinska J. Topical cyclosporine in high-risk corneal transplants. Ophthalmology. 1989 Aug;96(8):1144–1150. doi: 10.1016/s0161-6420(89)32756-4. [DOI] [PubMed] [Google Scholar]
- Boubenider S., Hiesse C., Goupy C., Kriaa F., Marchand S., Charpentier B. Incidence and consequences of post-transplantation lymphoproliferative disorders. J Nephrol. 1997 May-Jun;10(3):136–145. [PubMed] [Google Scholar]
- Bouchard C. S., Cavanagh H. D. The high-risk keratoplasty patient--quo vadis? Cornea. 1994 Jan;13(1):1–3. doi: 10.1097/00003226-199401000-00001. [DOI] [PubMed] [Google Scholar]
- Bourne W. M., Lindstrom R. L., Doughman D. J. Endothelial cell survival on transplanted human corneas preserved by organ culture with 1.35% chondroitin sulfate. Am J Ophthalmol. 1985 Dec 15;100(6):789–793. doi: 10.1016/s0002-9394(14)73368-9. [DOI] [PubMed] [Google Scholar]
- Frueh B. E., Böhnke M. Prospective, randomized clinical evaluation of Optisol vs organ culture corneal storage media. Arch Ophthalmol. 2000 Jun;118(6):757–760. doi: 10.1001/archopht.118.6.757. [DOI] [PubMed] [Google Scholar]
- Goichot-Bonnat L., Chemla P., Pouliquen Y. Cyclosporine A collyre dans la prévention du rejet de greffe de cornée à haut risque. II. Résultats cliniques post-opératoires. J Fr Ophtalmol. 1987;10(3):213–217. [PubMed] [Google Scholar]
- Hill J. C. Systemic cyclosporine in high-risk keratoplasty. Short- versus long-term therapy. Ophthalmology. 1994 Jan;101(1):128–133. doi: 10.1016/s0161-6420(13)31253-6. [DOI] [PubMed] [Google Scholar]
- Hill J. C. Systemic cyclosporine in high-risk keratoplasty: long-term results. Eye (Lond) 1995;9(Pt 4):422–428. doi: 10.1038/eye.1995.99. [DOI] [PubMed] [Google Scholar]
- Hoffmann F., Wiederholt M. Lokale Behandlung des Hornhauttransplantates beim Menschen mit Cyclosporin A. Klin Monbl Augenheilkd. 1985 Aug;187(2):92–96. doi: 10.1055/s-2008-1050995. [DOI] [PubMed] [Google Scholar]
- Holland E. J., Olsen T. W., Ketcham J. M., Florine C., Krachmer J. H., Purcell J. J., Lam S., Tessler H. H., Sugar J. Topical cyclosporin A in the treatment of anterior segment inflammatory disease. Cornea. 1993 Sep;12(5):413–419. doi: 10.1097/00003226-199309000-00008. [DOI] [PubMed] [Google Scholar]
- Leibowitz H. M. Management of inflammation in the cornea and conjunctiva. Ophthalmology. 1980 Aug;87(8):753–758. doi: 10.1016/s0161-6420(80)35174-9. [DOI] [PubMed] [Google Scholar]
- Lindstrom R. L., Kaufman H. E., Skelnik D. L., Laing R. A., Lass J. H., Musch D. C., Trousdale M. D., Reinhart W. J., Burris T. E., Sugar A. Optisol corneal storage medium. Am J Ophthalmol. 1992 Sep 15;114(3):345–356. doi: 10.1016/s0002-9394(14)71803-3. [DOI] [PubMed] [Google Scholar]
- Rijneveld W. J., Beekhuis W. H., van Rij G., Rinkel-van Driel B., Pels E. Clinical comparison of grafts stored in McCarey-Kaufman medium at 4 degrees C and in corneal organ culture at 31 degrees C. Arch Ophthalmol. 1992 Feb;110(2):203–205. doi: 10.1001/archopht.1992.01080140059027. [DOI] [PubMed] [Google Scholar]
- Robert P. Y., Delbosc B., Preux P. M., Monnot P. H., Drouet M., Peyronnet P., Adenis J. P. Traitement par ciclosporine A, à doses faibles, dans les kératoplasties transfixiantes à haut risque. Etude bicentrique de 90 cas. J Fr Ophtalmol. 1997;20(7):507–514. [PubMed] [Google Scholar]
- Roorda A., Williams D. R. The arrangement of the three cone classes in the living human eye. Nature. 1999 Feb 11;397(6719):520–522. doi: 10.1038/17383. [DOI] [PubMed] [Google Scholar]
- Sundmacher R., Reinhard T., Heering P. Six years' experience with systemic cyclosporin A prophylaxis in high-risk perforating keratoplasty patients. A retrospective study. Ger J Ophthalmol. 1992;1(6):432–436. [PubMed] [Google Scholar]
- Williams K. A., Muehlberg S. M., Lewis R. F., Coster D. J. Influence of advanced recipient and donor age on the outcome of corneal transplantation. Australian Corneal Graft Registry. Br J Ophthalmol. 1997 Oct;81(10):835–839. doi: 10.1136/bjo.81.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]