Abstract
AIM—To analyse the extent of bony orbital volume reduction after enucleation in humans. METHODS—Volumetric studies on bony orbital volumes based on three dimensional reconstructions acquired from high resolution computed tomograph (CT) scans were performed in 29 patients with acquired anophthalmia and four patients before enucleation (controls). Eight patients (follow up 25-52 years) were enucleated in childhood aged between 0.4 and 8 years (group I), 21 in adulthood aged between 15 and 53 years. Fifteen of these patients (group IIa) had long standing anophthalmia (follow up 7-53 years), six patients (group IIb) were enucleated 9 months to 4 years before CT. RESULTS—Bony orbital volumes were reduced in all patients with long standing anophthalmia. The median percentage reduction in enucleated orbits was 7.0% in group I, 3.8% in group IIa, and 1.9% in group IIb. In patients with long standing anophthalmia (I and IIa) the reductions were statistically significantly different (p <0.01) from zero. There was some evidence of a correlation between orbital volume reduction and age at enucleation (rho = 0.36, p = 0.09, Spearman rank correlation coefficient) and a statistically significant correlation between volume reduction and time interval since enucleation (rho = −0.5, p = 0.003). Clinically none of the patients showed significant facial asymmetry. CONCLUSIONS—These data provide strong evidence that enucleation both in children and adults is associated with a reduction of bony orbital volume and that this decrease in volume is associated with increasing time. However, the reduction is smaller than generally assumed and does not cause obvious facial asymmetry. It is more related to the time interval since enucleation than the age at enucleation, which makes a mechanism of volume adaptation more likely than just retardation of growth.
Full Text
The Full Text of this article is available as a PDF (149.5 KB).
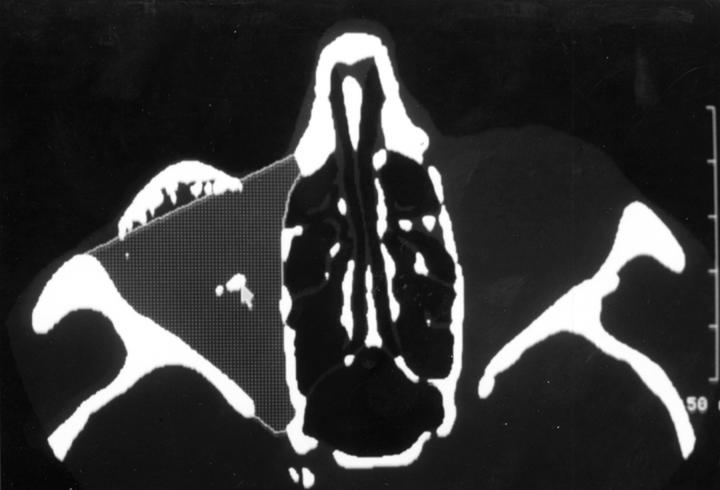
Figure 1 .
Segmentation of the right orbit. Grey areas compare with identical thresholds (min − 204 H/max +166 H). Stippled area outlined corresponds to the bony orbital volume. White dots (arrow) belong to the orbital implant.
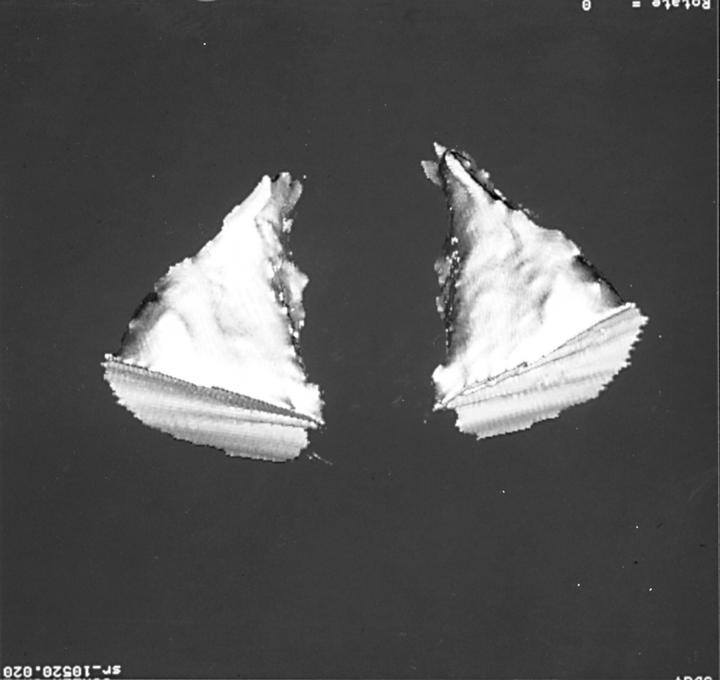
Figure 2 .
Bony orbital volume compartment, corresponding to an impression mould of the orbit. Thirty five year old patient, enucleated with primary orbital implant age 3.5 years. BOVenuc (right): 25.5 ml, BOV: 27.1 ml, BOV difference between enucleated and fellow orbit −5.5%.
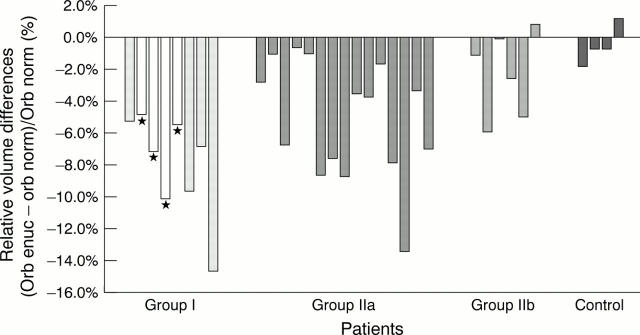
Figure 3 .
Bony orbital volume following enucleation. Relative volume differences, single values. Group I: childhood, long standing, group IIa: adulthood, long standing, group IIb: adulthood, short follow up. Negative values indicating smaller volume in the anophthalmic orbit. *Primary orbital implant.
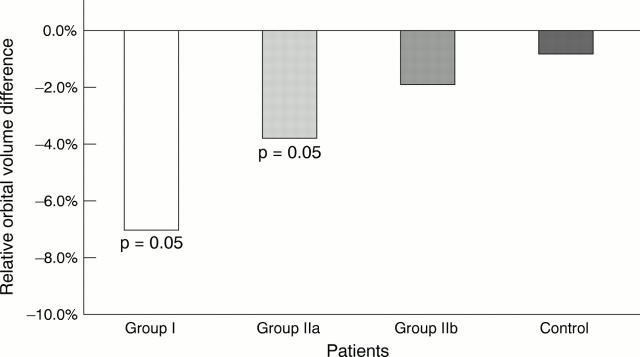
Figure 4 .
Bony orbital volume following enucleation. Relative volume differences, median.
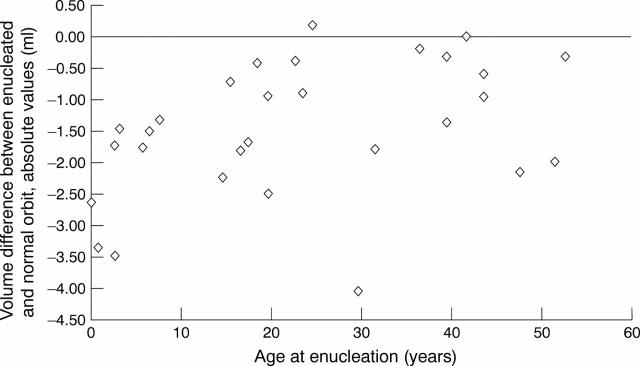
Figure 5 .
Scatter diagram. Age at enucleation versus BOV difference enucleated orbit − normal orbit. rho = 0.36, p = 0.09, Spearman rank order correlation.
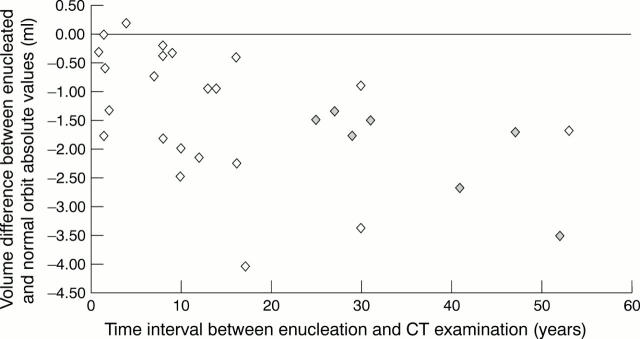
Figure 6 .
Scatter diagram. Time interval enucleation to CT examination versus BOV difference enucleated orbit − normal orbit. rho = −0.56, p = 0.003, Spearman rank order correlation. Grey diamond = patients enucleated in childhood.
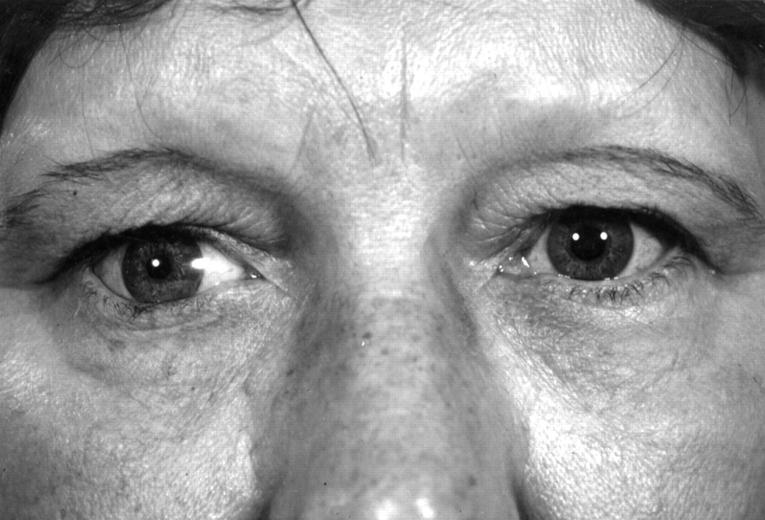
Figure 7 .
Fifty five year old patient enucleated at age 3 years, after secondary orbital implant. BOVenuc: 20.2 ml, BOV: 23.7 ml, BOV difference: −14.7%. No clinically obvious orbital malformation or facial asymmetry.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ameniya T., Matsumura M., Hirose Y. Effects of radiation after enucleation without implantation on orbital development of patients with retinoblastoma. Ophthalmologica. 1977;174(3):137–144. doi: 10.1159/000308591. [DOI] [PubMed] [Google Scholar]
- Apt L., Isenberg S. Changes in orbital dimensions following enucleation. Arch Ophthalmol. 1973 Nov;90(5):393–395. doi: 10.1001/archopht.1973.01000050395013. [DOI] [PubMed] [Google Scholar]
- Cepela M. A., Nunery W. R., Martin R. T. Stimulation of orbital growth by the use of expandable implants in the anophthalmic cat orbit. Ophthal Plast Reconstr Surg. 1992;8(3):157–169. doi: 10.1097/00002341-199209000-00001. [DOI] [PubMed] [Google Scholar]
- Eppley B. L., Holley S., Sadove A. M. Experimental effects of intraorbital tissue expansion on orbitomaxillary growth in anophthalmos. Ann Plast Surg. 1993 Jul;31(1):19–27. [PubMed] [Google Scholar]
- Fountain T. R., Goldberger S., Murphree A. L. Orbital development after enucleation in early childhood. Ophthal Plast Reconstr Surg. 1999 Jan;15(1):32–36. doi: 10.1097/00002341-199901000-00008. [DOI] [PubMed] [Google Scholar]
- HOWARD G. M., KINDER R. S., MACMILLAN A. S., Jr ORBITAL GROWTH AFTER UNILATERAL ENUCLEATION IN CHILDHOOD. Arch Ophthalmol. 1965 Jan;73:80–83. doi: 10.1001/archopht.1965.00970030082016. [DOI] [PubMed] [Google Scholar]
- Heinz G. W., Nunery W. R., Cepela M. A. The effect of maturation on the ability to stimulate orbital growth using tissue expanders in the anophthalmic cat orbit. Ophthal Plast Reconstr Surg. 1997 Jun;13(2):115–128. doi: 10.1097/00002341-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Honegger H., Müller-Staufenbiel H. Das Wachstum der Orbita nach Enukleation im frühen Kindesalter und ihr Einfluss auf das kosmetische Spätergebnis. Klin Monbl Augenheilkd. 1967;150(5):655–663. [PubMed] [Google Scholar]
- KENNEDY R. E. THE EFFECT OF EARLY ENUCLEATION ON THE ORBIT IN ANIMALS AND HUMANS. Trans Am Ophthalmol Soc. 1964;62:459–510. [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. E. Bone changes in the adult anophthalmic orbit influencing oculoplastic reconstructive considerations. Trans Am Ophthalmol Soc. 1976;74:237–250. [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. E. The effect of early enucleation on the orbit in animals and humans. Adv Ophthalmic Plast Reconstr Surg. 1992;9:1–39. [PubMed] [Google Scholar]
- Osborne D., Hadden O. B., Deeming L. W. Orbital growth after childhood enucleation. Am J Ophthalmol. 1974 May;77(5):756–759. doi: 10.1016/0002-9394(74)90545-5. [DOI] [PubMed] [Google Scholar]
- SARNAT B. G., SHANEDLING P. D. POSTNATAL GROWTH OF THE ORBIT AND UPPER FACE IN RABBITS AFTER EXENTERATION OF THE ORBIT. Arch Ophthalmol. 1965 Jun;73:829–837. doi: 10.1001/archopht.1965.00970030831015. [DOI] [PubMed] [Google Scholar]
- Sarnat B. G. Eye volume in young and adult rabbits. Acta Anat (Basel) 1980;106(4):462–467. doi: 10.1159/000145215. [DOI] [PubMed] [Google Scholar]
- Sarnat B. G. Orbital volume after enucleation and eye volume in the adult rabbit. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978 Nov 30;208(4):241–245. doi: 10.1007/BF00419379. [DOI] [PubMed] [Google Scholar]
- van Limborgh J., Tonneyck-Müller I. Orbital growth pattern in experimental microphthalmia. Mod Probl Ophthalmol. 1975;14:1–4. [PubMed] [Google Scholar]