Abstract
BACKGROUND/AIMS—Retinal degenerations are a leading cause of blindness for which there are currently no effective treatments. This has stimulated interest in the investigation of gene therapy strategies for these diseases in a variety of animal models. A number of attempts have been made to prevent photoreceptor loss in the rd mouse model of retinal degeneration using adenoviral vectors containing either a copy of the missing functional gene or a gene encoding either a neurotrophic factor or an antiapoptotic factor. The authors have previously demonstrated that intraocular administration of an adenoviral vector containing a β galactosidase gene (AV.LacZ) results in an immune response to viral gene products and β galactosidase. Here the effect of the immune response on retinal degeneration is examined. METHODS—Juvenile rd mice were injected intravitreally with AV.LacZ and a proportion were depleted of either CD4+ or CD8+ T cells or both. Control animals were injected with PBS. The mice were sacrificed 10 and 20 days post-injection and their eyes embedded in paraffin wax and sectioned. RESULTS—10 days after intravitreal injection of AV.LacZ, the outer nuclear layer contains an average of 2.5 rows compared with 1.5 in PBS injected animals (p<0.005). The protective effect of AV.LacZ is negated by immune suppression and does not extend beyond 20 days. CONCLUSION—An immune response to vector and transgene products is able to slow degeneration in the rd mouse. This phenomenon should be taken into account when analysing the degeneration in the rd mouse following gene transfer.
Full Text
The Full Text of this article is available as a PDF (178.9 KB).
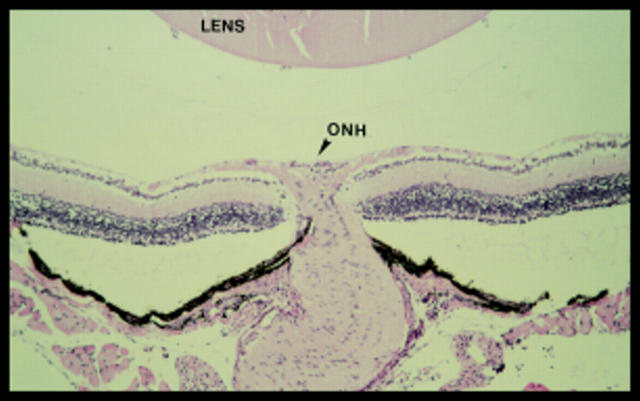
Figure 1 .
Eyes were sectioned to the level of optic nerve head (ONH) and only sections taken close to either side of the ONH were evaluated to ensure that retinas of normally equivalent thicknesses were compared. A 5 µm paraffin wax section counterstained with haematoxylin and eosin (10× objective) taken from 22 day old rd mouse 10 days after intravitreal injection of 1 µl of PBS.
Figure 2 .
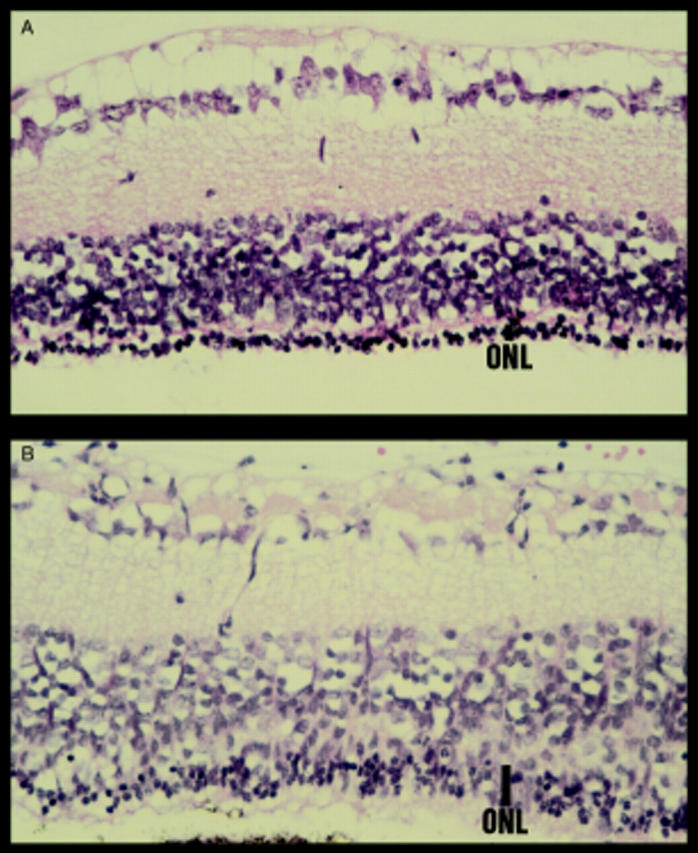
Examples of sections taken close to the ONH region of 22 day old rd mice 10 days after intravitreal injection of either (A) 1 µl of PBS or (B) 1 µl of AV.LacZ (1×106 pfu). The 5 µm paraffin sections were counterstained with haematoxylin and eosin (40× objective). Eyes injected with PBS had between one and two rows of photoreceptors in the outer nuclear layer (ONL) whereas in some eyes injected with AV.Lac Z there were between three and four rows of photoreceptors.
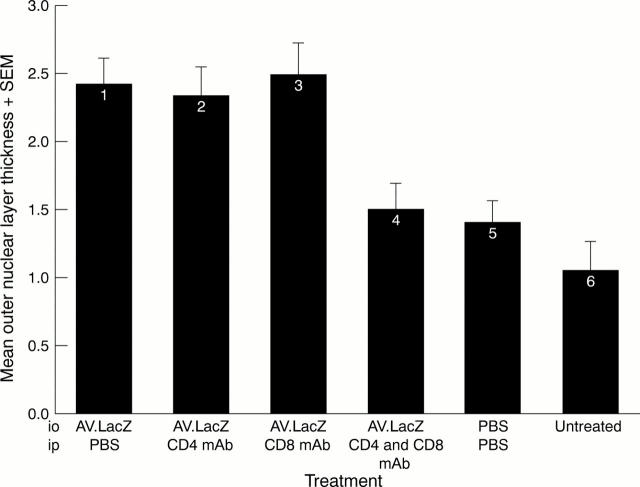
Figure 3 .
Comparison of rd ONL thickness in six groups of animals following intraocular injection (io) of either PBS or AV.LacZ with and without T cell ablation as a result of intraperitoneal injection (ip) of either PBS or monoclonal antibodies (mAb) specific for CD4 or CD8. The number of eyes in each treatment group (from 1 to 6) is 12, 6, 6, 8, 10, and 10 respectively. The scoring system was as follows: 0, a sparse row of photoreceptors; 1, a single row of photoreceptors; 2, between one and two rows of photoreceptors; 3, between two and three rows of photoreceptors, and so on. The data show the standard error of the mean ONL score. The difference in ONL thickness between treatment groups 1, 2, and 3 is not statistically significant. There are also no statistically significant differences in ONL thickness between treatment groups 4, 5, and 6. There is, however, a significant difference between groups 1 and 4 (p> 0.01); 1 and 5 (p< 0.005); 1 and 6 (p< 0.001); 2 and 4 (p< 0.05); 2 and 5 (p<0.05); 2 and 6 (p<0.005); 3 and 4 (p<0.05); 3 and 5 (p<0.05); 3 and 6 (p<0.005).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascom R. A., Liu L., Heckenlively J. R., Stone E. M., McInnes R. R. Mutation analysis of the ROM1 gene in retinitis pigmentosa. Hum Mol Genet. 1995 Oct;4(10):1895–1902. doi: 10.1093/hmg/4.10.1895. [DOI] [PubMed] [Google Scholar]
- Bennett J., Wilson J., Sun D., Forbes B., Maguire A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci. 1994 Apr;35(5):2535–2542. [PubMed] [Google Scholar]
- Bennett J., Zeng Y., Bajwa R., Klatt L., Li Y., Maguire A. M. Adenovirus-mediated delivery of rhodopsin-promoted bcl-2 results in a delay in photoreceptor cell death in the rd/rd mouse. Gene Ther. 1998 Sep;5(9):1156–1164. doi: 10.1038/sj.gt.3300733. [DOI] [PubMed] [Google Scholar]
- Bessant D. A., Payne A. M., Mitton K. P., Wang Q. L., Swain P. K., Plant C., Bird A. C., Zack D. J., Swaroop A., Bhattacharya S. S. A mutation in NRL is associated with autosomal dominant retinitis pigmentosa. Nat Genet. 1999 Apr;21(4):355–356. doi: 10.1038/7678. [DOI] [PubMed] [Google Scholar]
- Bird A. C. Retinal photoreceptor dystrophies LI. Edward Jackson Memorial Lecture. Am J Ophthalmol. 1995 May;119(5):543–562. doi: 10.1016/s0002-9394(14)70212-0. [DOI] [PubMed] [Google Scholar]
- Byrnes A. P., Rusby J. E., Wood M. J., Charlton H. M. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995 Jun;66(4):1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- Cao W., Wen R., Li F., Lavail M. M., Steinberg R. H. Mechanical injury increases bFGF and CNTF mRNA expression in the mouse retina. Exp Eye Res. 1997 Aug;65(2):241–248. doi: 10.1006/exer.1997.0328. [DOI] [PubMed] [Google Scholar]
- Cayouette M., Gravel C. Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Hum Gene Ther. 1997 Mar 1;8(4):423–430. doi: 10.1089/hum.1997.8.4-423. [DOI] [PubMed] [Google Scholar]
- Di Polo A., Aigner L. J., Dunn R. J., Bray G. M., Aguayo A. J. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Müller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Finn J. T., Peng Y. W., McGee T. L., Berson E. L., Yau K. W. Mutations in the gene encoding the alpha subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10177–10181. doi: 10.1073/pnas.92.22.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990 Jan 25;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Farrar G. J., Kenna P., Jordan S. A., Kumar-Singh R., Humphries M. M., Sharp E. M., Sheils D. M., Humphries P. A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):478–480. doi: 10.1038/354478a0. [DOI] [PubMed] [Google Scholar]
- Freund C. L., Gregory-Evans C. Y., Furukawa T., Papaioannou M., Looser J., Ploder L., Bellingham J., Ng D., Herbrick J. A., Duncan A. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell. 1997 Nov 14;91(4):543–553. doi: 10.1016/s0092-8674(00)80440-7. [DOI] [PubMed] [Google Scholar]
- Jomary C., Vincent K. A., Grist J., Neal M. J., Jones S. E. Rescue of photoreceptor function by AAV-mediated gene transfer in a mouse model of inherited retinal degeneration. Gene Ther. 1997 Jul;4(7):683–690. doi: 10.1038/sj.gt.3300440. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M., Gallmeier E., Behrens L., Leal V. V., Misgeld T., Klinkert W. E., Kolbeck R., Hoppe E., Oropeza-Wekerle R. L., Bartke I. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999 Mar 1;189(5):865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh R., Farber D. B. Encapsidated adenovirus mini-chromosome-mediated delivery of genes to the retina: application to the rescue of photoreceptor degeneration. Hum Mol Genet. 1998 Nov;7(12):1893–1900. doi: 10.1093/hmg/7.12.1893. [DOI] [PubMed] [Google Scholar]
- McLaughlin M. E., Sandberg M. A., Berson E. L., Dryja T. P. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993 Jun;4(2):130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- Moalem G., Leibowitz-Amit R., Yoles E., Mor F., Cohen I. R., Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999 Jan;5(1):49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Mohand-Said S., Deudon-Combe A., Hicks D., Simonutti M., Forster V., Fintz A. C., Léveillard T., Dreyfus H., Sahel J. A. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc Natl Acad Sci U S A. 1998 Jul 7;95(14):8357–8362. doi: 10.1073/pnas.95.14.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohand-Said S., Hicks D., Simonutti M., Tran-Minh D., Deudon-Combe A., Dreyfus H., Silverman M. S., Ogilvie J. M., Tenkova T., Sahel J. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res. 1997;29(5):290–297. doi: 10.1159/000268027. [DOI] [PubMed] [Google Scholar]
- O'Neill J. K., Baker D., Davison A. N., Allen S. J., Butter C., Waldmann H., Turk J. L. Control of immune-mediated disease of the central nervous system with monoclonal (CD4-specific) antibodies. J Neuroimmunol. 1993 Jun;45(1-2):1–14. doi: 10.1016/0165-5728(93)90157-t. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C., Sung C. H., Nathans J., Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):974–978. doi: 10.1073/pnas.91.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel M. B., Ali R. R., Thrasher A. J., Hunt D. M., Bhattacharya S. S., Baker D. Immune responses limit adenovirally mediated gene expression in the adult mouse eye. Gene Ther. 1998 Aug;5(8):1038–1046. doi: 10.1038/sj.gt.3300691. [DOI] [PubMed] [Google Scholar]
- Wen R., Song Y., Cheng T., Matthes M. T., Yasumura D., LaVail M. M., Steinberg R. H. Injury-induced upregulation of bFGF and CNTF mRNAS in the rat retina. J Neurosci. 1995 Nov;15(11):7377–7385. doi: 10.1523/JNEUROSCI.15-11-07377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]