Full Text
The Full Text of this article is available as a PDF (138.4 KB).
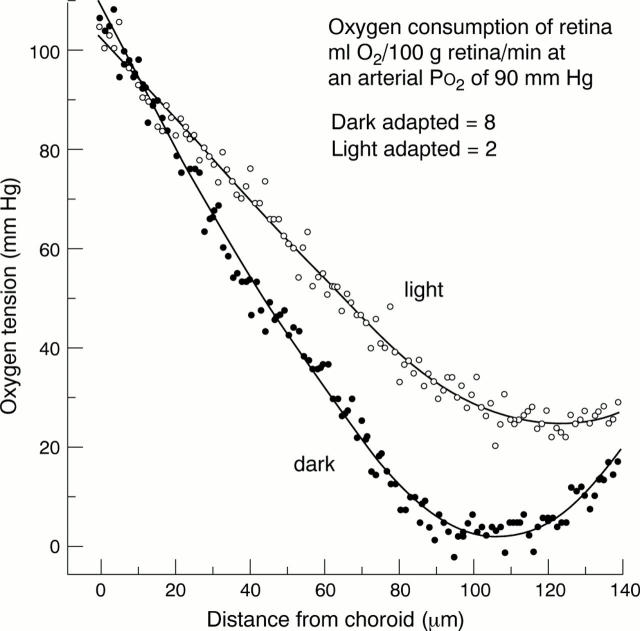
Figure 1 .
Relation between retinal depth and oxygen tension in darkness and light. Redrawn from refs 5-7.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed J., Braun R. D., Dunn R., Jr, Linsenmeier R. A. Oxygen distribution in the macaque retina. Invest Ophthalmol Vis Sci. 1993 Mar;34(3):516–521. [PubMed] [Google Scholar]
- Aiello L. P., Avery R. L., Arrigg P. G., Keyt B. A., Jampel H. D., Shah S. T., Pasquale L. R., Thieme H., Iwamoto M. A., Park J. E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994 Dec 1;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Aiello L. P., Bursell S. E., Clermont A., Duh E., Ishii H., Takagi C., Mori F., Ciulla T. A., Ways K., Jirousek M. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997 Sep;46(9):1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- Alder V. A., Yu D. Y., Cringle S. J., Su E. N. Changes in vitreal oxygen tension distribution in the streptozotocin diabetic rat. Diabetologia. 1991 Jul;34(7):469–476. doi: 10.1007/BF00403282. [DOI] [PubMed] [Google Scholar]
- Amemiya T. Dark adaptation in diabetics. Ophthalmologica. 1977;174(6):322–326. doi: 10.1159/000308622. [DOI] [PubMed] [Google Scholar]
- Amin R. H., Frank R. N., Kennedy A., Eliott D., Puklin J. E., Abrams G. W. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997 Jan;38(1):36–47. [PubMed] [Google Scholar]
- Antonetti D. A., Barber A. J., Hollinger L. A., Wolpert E. B., Gardner T. W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999 Aug 13;274(33):23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- Antonetti D. A., Barber A. J., Khin S., Lieth E., Tarbell J. M., Gardner T. W. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998 Dec;47(12):1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- Arden G. B., Wolf J. E., Tsang Y. Does dark adaptation exacerbate diabetic retinopathy? Evidence and a linking hypothesis. Vision Res. 1998 Jun;38(11):1723–1729. doi: 10.1016/s0042-6989(98)00004-2. [DOI] [PubMed] [Google Scholar]
- Beetham W. P., Aiello L. M., Balodimos M. C., Koncz L. Ruby laser photocoagulation of early diabetic neovascular retinopathy. Preliminary report of a long-term controlled study. Arch Ophthalmol. 1970 Mar;83(3):261–272. doi: 10.1001/archopht.1970.00990030263001. [DOI] [PubMed] [Google Scholar]
- Behzadian M. A., Wang X. L., Al-Shabrawey M., Shabrawey M., Caldwell R. B. Effects of hypoxia on glial cell expression of angiogenesis-regulating factors VEGF and TGF-beta. Glia. 1998 Oct;24(2):216–225. [PubMed] [Google Scholar]
- Bessant D. A., Payne A. M., Mitton K. P., Wang Q. L., Swain P. K., Plant C., Bird A. C., Zack D. J., Swaroop A., Bhattacharya S. S. A mutation in NRL is associated with autosomal dominant retinitis pigmentosa. Nat Genet. 1999 Apr;21(4):355–356. doi: 10.1038/7678. [DOI] [PubMed] [Google Scholar]
- Boulton M., Foreman D., Williams G., McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998 May;82(5):561–568. doi: 10.1136/bjo.82.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. D., Linsenmeier R. A., Goldstick T. K. Oxygen consumption in the inner and outer retina of the cat. Invest Ophthalmol Vis Sci. 1995 Mar;36(3):542–554. [PubMed] [Google Scholar]
- Bressler N. M., Gragoudas E. S. Neovascularization of the optic disk associated with atypical retinitis pigmentosa. Am J Ophthalmol. 1985 Sep 15;100(3):431–433. doi: 10.1016/0002-9394(85)90507-0. [DOI] [PubMed] [Google Scholar]
- Buraczynska M., Wu W., Fujita R., Buraczynska K., Phelps E., Andréasson S., Bennett J., Birch D. G., Fishman G. A., Hoffman D. R. Spectrum of mutations in the RPGR gene that are identified in 20% of families with X-linked retinitis pigmentosa. Am J Hum Genet. 1997 Dec;61(6):1287–1292. doi: 10.1086/301646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursell S. E., Clermont A. C., Kinsley B. T., Simonson D. C., Aiello L. M., Wolpert H. A. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996 Apr;37(5):886–897. [PubMed] [Google Scholar]
- Cringle S. J., Yu D. Y., Alder V. A., Su E. N., Yu P. K. Quantification of retinal oxygen consumption changes from preretinal oxygen transients. Aust N Z J Ophthalmol. 1998 May;26 (Suppl 1):S71–S73. doi: 10.1111/j.1442-9071.1998.tb01379.x. [DOI] [PubMed] [Google Scholar]
- Cringle S. J., Yu D. Y., Alder V., Su E. N. Light and choroidal PO2 modulation of intraretinal oxygen levels in an avascular retina. Invest Ophthalmol Vis Sci. 1999 Sep;40(10):2307–2313. [PubMed] [Google Scholar]
- Dean F. M., Arden G. B., Dornhorst A. Partial reversal of protan and tritan colour defects with inhaled oxygen in insulin dependent diabetic subjects. Br J Ophthalmol. 1997 Jan;81(1):27–30. doi: 10.1136/bjo.81.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzel J., Standl E. The oxygen transport system of red blood cells during diabetic ketoacidosis and recovery. Diabetologia. 1975 Aug;11(4):255–260. doi: 10.1007/BF00422388. [DOI] [PubMed] [Google Scholar]
- Dryja T. P. Gene-based approach to human gene-phenotype correlations. Proc Natl Acad Sci U S A. 1997 Oct 28;94(22):12117–12121. doi: 10.1073/pnas.94.22.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh E., Aiello L. P. Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes. 1999 Oct;48(10):1899–1906. doi: 10.2337/diabetes.48.10.1899. [DOI] [PubMed] [Google Scholar]
- Ellis E. A., Guberski D. L., Somogyi-Mann M., Grant M. B. Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med. 2000 Jan 1;28(1):91–101. doi: 10.1016/s0891-5849(99)00216-6. [DOI] [PubMed] [Google Scholar]
- Favard C., Ortega N., Bayard F., Plouet J. Vascular endothelial growth factor and retinal neovascularisation: a new therapeutic approach for diabetic retinopathy. Diabetes Metab. 1996 Jul;22(4):268–273. [PubMed] [Google Scholar]
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med (Berl) 1999 Jul;77(7):527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- Fishman G. A., Grover S., Jacobson S. G., Alexander K. R., Derlacki D. J., Wu W., Buraczynska M., Swaroop A. X-linked retinitis pigmentosa in two families with a missense mutation in the RPGR gene and putative change of glycine to valine at codon 60. Ophthalmology. 1998 Dec;105(12):2286–2296. doi: 10.1016/S0161-6420(98)91231-3. [DOI] [PubMed] [Google Scholar]
- Gerhardinger C., Brown L. F., Roy S., Mizutani M., Zucker C. L., Lorenzi M. Expression of vascular endothelial growth factor in the human retina and in nonproliferative diabetic retinopathy. Am J Pathol. 1998 Jun;152(6):1453–1462. [PMC free article] [PubMed] [Google Scholar]
- Gilbert R. E., Vranes D., Berka J. L., Kelly D. J., Cox A., Wu L. L., Stacker S. A., Cooper M. E. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab Invest. 1998 Aug;78(8):1017–1027. [PubMed] [Google Scholar]
- Goldstick T. K., Ernest J. T., Engerman R. L. Retinal oxygen tension in diabetic dogs following insulin infusion. Adv Exp Med Biol. 1984;169:661–670. doi: 10.1007/978-1-4684-1188-1_60. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Ross P. D., Tate R. L., Yoshikami S. Transduction heats in retinal rods: tests of the role of cGMP by pyroelectric calorimetry. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1224–1228. doi: 10.1073/pnas.86.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes H. P., Lin J., Bretzel R. G., Brownlee M., Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998 Mar;47(3):401–406. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- Harris A., Arend O., Danis R. P., Evans D., Wolf S., Martin B. J. Hyperoxia improves contrast sensitivity in early diabetic retinopathy. Br J Ophthalmol. 1996 Mar;80(3):209–213. doi: 10.1136/bjo.80.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh-Scheidt L. M., Griff E. R., Linsenmeier R. A. Light-evoked oxygen responses in the isolated toad retina. Exp Eye Res. 1995 Jul;61(1):73–81. doi: 10.1016/s0014-4835(95)80060-3. [DOI] [PubMed] [Google Scholar]
- Haugh L. M., Linsenmeier R. A., Goldstick T. K. Mathematical models of the spatial distribution of retinal oxygen tension and consumption, including changes upon illumination. Ann Biomed Eng. 1990;18(1):19–36. doi: 10.1007/BF02368415. [DOI] [PubMed] [Google Scholar]
- Havelius U., Berglund S., Falke P., Hindfelt B., Krakau T. Impaired dark adaptation in polycythemia. Improvement after treatment. Acta Ophthalmol Scand. 2000 Feb;78(1):53–57. doi: 10.1034/j.1600-0420.2000.078001053.x. [DOI] [PubMed] [Google Scholar]
- Havelius U., Bergqvist D., Falke P., Hindfelt B., Krakau T. I. Impaired dark adaptation in symptomatic carotid artery disease. Neurology. 1997 Nov;49(5):1353–1359. doi: 10.1212/wnl.49.5.1353. [DOI] [PubMed] [Google Scholar]
- Havelius U., Bergqvist D., Hindfelt B., Krakau T. II. Improved dark adaptation after carotid endarterectomy. Evidence of a long-term ischemic penumbra? Neurology. 1997 Nov;49(5):1360–1364. doi: 10.1212/wnl.49.5.1360. [DOI] [PubMed] [Google Scholar]
- Holmes-Walker D. J., Mitchell P., Boyages S. C. Does mitochondrial genome mutation in subjects with maternally inherited diabetes and deafness decrease severity of diabetic retinopathy? Diabet Med. 1998 Nov;15(11):946–952. doi: 10.1002/(SICI)1096-9136(1998110)15:11<946::AID-DIA707>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Huang S. H., Pittler S. J., Huang X., Oliveira L., Berson E. L., Dryja T. P. Autosomal recessive retinitis pigmentosa caused by mutations in the alpha subunit of rod cGMP phosphodiesterase. Nat Genet. 1995 Dec;11(4):468–471. doi: 10.1038/ng1295-468. [DOI] [PubMed] [Google Scholar]
- Inglehearn C. F. Molecular genetics of human retinal dystrophies. Eye (Lond) 1998;12(Pt 3B):571–579. doi: 10.1038/eye.1998.147. [DOI] [PubMed] [Google Scholar]
- Janecke A. R., Meins M., Sadeghi M., Grundmann K., Apfelstedt-Sylla E., Zrenner E., Rosenberg T., Gal A. Twelve novel myosin VIIA mutations in 34 patients with Usher syndrome type I: confirmation of genetic heterogeneity. Hum Mutat. 1999;13(2):133–140. doi: 10.1002/(SICI)1098-1004(1999)13:2<133::AID-HUMU5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Jurklies B., Zrenner E., Wessing A. Retinitis pigmentosa--klinische, genetische und pathophysiologische Aspekte. Klin Monbl Augenheilkd. 1997 Jan;210(1):1–18. doi: 10.1055/s-2008-1035006. [DOI] [PubMed] [Google Scholar]
- Kern T. S., Engerman R. L. Capillary lesions develop in retina rather than cerebral cortex in diabetes and experimental galactosemia. Arch Ophthalmol. 1996 Mar;114(3):306–310. doi: 10.1001/archopht.1996.01100130302013. [DOI] [PubMed] [Google Scholar]
- Khaliq A., Jarvis-Evans J., McLeod D., Boulton M. Oxygen modulates the response of the retinal pigment epithelium to basic fibroblast growth factor and epidermal growth factor by receptor regulation. Invest Ophthalmol Vis Sci. 1996 Feb;37(2):436–443. [PubMed] [Google Scholar]
- Kirschner R., Rosenberg T., Schultz-Heienbrok R., Lenzner S., Feil S., Roepman R., Cremers F. P., Ropers H. H., Berger W. RPGR transcription studies in mouse and human tissues reveal a retina-specific isoform that is disrupted in a patient with X-linked retinitis pigmentosa. Hum Mol Genet. 1999 Aug;8(8):1571–1578. doi: 10.1093/hmg/8.8.1571. [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B. E., Moss S. E., Davis M. D., DeMets D. L. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984 Apr;102(4):527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- Konno S., Feke G. T., Yoshida A., Fujio N., Goger D. G., Buzney S. M. Retinal blood flow changes in type I diabetes. A long-term follow-up study. Invest Ophthalmol Vis Sci. 1996 May;37(6):1140–1148. [PubMed] [Google Scholar]
- Kucinskas V., Payne A. M., Ambrasiene D., Jurgelevicius V., Steponaviciūte D., Arciuliene J. V., Daktaraviciene E., Bhattacharya S. Molecular genetic study of autosomal dominant retinitis pigmentosa in Lithuanian patients. Hum Hered. 1999 Mar;49(2):71–74. doi: 10.1159/000022847. [DOI] [PubMed] [Google Scholar]
- Linsenmeier R. A., Braun R. D., McRipley M. A., Padnick L. B., Ahmed J., Hatchell D. L., McLeod D. S., Lutty G. A. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci. 1998 Aug;39(9):1647–1657. [PubMed] [Google Scholar]
- Linsenmeier R. A., Braun R. D. Oxygen distribution and consumption in the cat retina during normoxia and hypoxemia. J Gen Physiol. 1992 Feb;99(2):177–197. doi: 10.1085/jgp.99.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier R. A. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986 Oct;88(4):521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Amano S., Miyamoto K., Garland R., Keough K., Qin W., Adamis A. P. Insulin-induced vascular endothelial growth factor expression in retina. Invest Ophthalmol Vis Sci. 1999 Dec;40(13):3281–3286. [PubMed] [Google Scholar]
- Lu M., Kuroki M., Amano S., Tolentino M., Keough K., Kim I., Bucala R., Adamis A. P. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J Clin Invest. 1998 Mar 15;101(6):1219–1224. doi: 10.1172/JCI1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Perez V. L., Ma N., Miyamoto K., Peng H. B., Liao J. K., Adamis A. P. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci. 1999 Jul;40(8):1808–1812. [PubMed] [Google Scholar]
- Marsh S., Nakhoul F. M., Skorecki K., Rubin A., Miller B. P., Leibu R., Levy N. S., Levy A. P. Hypoxic induction of vascular endothelial growth factor is markedly decreased in diabetic individuals who do not develop retinopathy. Diabetes Care. 2000 Sep;23(9):1375–1380. doi: 10.2337/diacare.23.9.1375. [DOI] [PubMed] [Google Scholar]
- Mathews M. K., Merges C., McLeod D. S., Lutty G. A. Vascular endothelial growth factor and vascular permeability changes in human diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997 Dec;38(13):2729–2741. [PubMed] [Google Scholar]
- Maw M. A., Kennedy B., Knight A., Bridges R., Roth K. E., Mani E. J., Mukkadan J. K., Nancarrow D., Crabb J. W., Denton M. J. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat Genet. 1997 Oct;17(2):198–200. doi: 10.1038/ng1097-198. [DOI] [PubMed] [Google Scholar]
- Millá E., Héon E., Grounauer P. A., Piguet B., Ducrey N., Stone E. M., Schorderet D. F., Munier F. L. Rhodopsin C110Y mutation causes a type 2 autosomal dominant retinitis pigmentosa. Ophthalmic Genet. 1998 Sep;19(3):131–139. doi: 10.1076/opge.19.3.131.2183. [DOI] [PubMed] [Google Scholar]
- Millá E., Héon E., Piguet B., Ducrey N., Butler N., Stone E., Schorderet D. F., Munier F. Depistage mutationnel des genes de la peripherine/RDS, rhodopsine et ROM-1 dans 69 cas index de retinite pigmentaire et autres dystrophies retiniennes. Klin Monbl Augenheilkd. 1998 May;212(5):305–308. [PubMed] [Google Scholar]
- Mitchell P., Moffitt P. Update and implications from the Newcastle diabetic retinopathy study. Aust N Z J Ophthalmol. 1990 Feb;18(1):13–17. doi: 10.1111/j.1442-9071.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Mizutani M., Gerhardinger C., Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998 Mar;47(3):445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- Murata T., Nakagawa K., Khalil A., Ishibashi T., Inomata H., Sueishi K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest. 1996 Apr;74(4):819–825. [PubMed] [Google Scholar]
- Nakazawa M., Wada Y., Tamai M. Arrestin gene mutations in autosomal recessive retinitis pigmentosa. Arch Ophthalmol. 1998 Apr;116(4):498–501. doi: 10.1001/archopht.116.4.498. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Seo M. S., Ozaki K., Yamada H., Yamada E., Okamoto N., Hofmann F., Wood J. M., Campochiaro P. A. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000 Feb;156(2):697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V., Rassam S., Newsom R., Wiek J., Kohner E. Retinal blood flow in diabetic retinopathy. BMJ. 1992 Sep 19;305(6855):678–683. doi: 10.1136/bmj.305.6855.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V., Rassam S., Newsom R., Wiek J., Kohner E. Retinal blood flow in diabetic retinopathy. BMJ. 1992 Sep 19;305(6855):678–683. doi: 10.1136/bmj.305.6855.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponjavic V., Abrahamson M., Andréasson S., Ehinger B., Fex G. Autosomal dominant retinitis pigmentosa with a rhodopsin mutation (Arg-135-Trp). Disease phenotype in a Swedish family. Acta Ophthalmol Scand. 1997 Apr;75(2):218–223. doi: 10.1111/j.1600-0420.1997.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Ponjavic V., Abrahamson M., Andréasson S., Ehinger B., Fex G., Polland W. A mild phenotype of autosomal dominant retinitis pigmentosa is associated with the rhodopsin mutation Pro-267-Leu. Ophthalmic Genet. 1997 Jun;18(2):63–70. [PubMed] [Google Scholar]
- Pécsvarády Z., Fisher T. C., Darwin C. H., Fabók A., Maqueda T. S., Saad M. F., Meiselman H. J. Decreased polymorphonuclear leukocyte deformability in NIDDM. Diabetes Care. 1994 Jan;17(1):57–63. doi: 10.2337/diacare.17.1.57. [DOI] [PubMed] [Google Scholar]
- Segawa Y., Shirao Y., Yamagishi S., Higashide T., Kobayashi M., Katsuno K., Iyobe A., Harada H., Sato F., Miyata H. Upregulation of retinal vascular endothelial growth factor mRNAs in spontaneously diabetic rats without ophthalmoscopic retinopathy. A possible participation of advanced glycation end products in the development of the early phase of diabetic retinopathy. Ophthalmic Res. 1998;30(6):333–339. doi: 10.1159/000055493. [DOI] [PubMed] [Google Scholar]
- Shinoda K., Ishida S., Kawashima S., Wakabayashi T., Matsuzaki T., Takayama M., Shinmura K., Yamada M. Comparison of the levels of hepatocyte growth factor and vascular endothelial growth factor in aqueous fluid and serum with grades of retinopathy in patients with diabetes mellitus. Br J Ophthalmol. 1999 Jul;83(7):834–837. doi: 10.1136/bjo.83.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G., McLeod D., Foreman D., Boulton M. Immunolocalisation of the VEGF receptors FLT-1, KDR, and FLT-4 in diabetic retinopathy. Br J Ophthalmol. 1999 Apr;83(4):486–494. doi: 10.1136/bjo.83.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefánsson E., Hatchell D. L., Fisher B. L., Sutherland F. S., Machemer R. Panretinal photocoagulation and retinal oxygenation in normal and diabetic cats. Am J Ophthalmol. 1986 Jun 15;101(6):657–664. doi: 10.1016/0002-9394(86)90765-8. [DOI] [PubMed] [Google Scholar]
- Stefánsson E., Hatchell D. L., Fisher B. L., Sutherland F. S., Machemer R. Panretinal photocoagulation and retinal oxygenation in normal and diabetic cats. Am J Ophthalmol. 1986 Jun 15;101(6):657–664. doi: 10.1016/0002-9394(86)90765-8. [DOI] [PubMed] [Google Scholar]
- Stefánsson E. Retinal oxygen tension is higher in light than dark. Pediatr Res. 1988 Jan;23(1):5–8. doi: 10.1203/00006450-198801000-00002. [DOI] [PubMed] [Google Scholar]
- Stefánsson E. Retinal oxygen tension is higher in light than dark. Pediatr Res. 1988 Jan;23(1):5–8. doi: 10.1203/00006450-198801000-00002. [DOI] [PubMed] [Google Scholar]
- Sternberg P., Jr, Landers M. B., 3rd, Wolbarsht M. The negative coincidence of retinitis pigmentosa and proliferative diabetic retinopathy. Am J Ophthalmol. 1984 Jun;97(6):788–789. doi: 10.1016/0002-9394(84)90518-x. [DOI] [PubMed] [Google Scholar]
- Sue C. M., Mitchell P., Crimmins D. S., Moshegov C., Byrne E., Morris J. G. Pigmentary retinopathy associated with the mitochondrial DNA 3243 point mutation. Neurology. 1997 Oct;49(4):1013–1017. doi: 10.1212/wnl.49.4.1013. [DOI] [PubMed] [Google Scholar]
- Tillis T. N., Murray D. L., Schmidt G. J., Weiter J. J. Preretinal oxygen changes in the rabbit under conditions of light and dark. Invest Ophthalmol Vis Sci. 1988 Jun;29(6):988–991. [PubMed] [Google Scholar]
- Tillis T. N., Murray D. L., Schmidt G. J., Weiter J. J. Preretinal oxygen changes in the rabbit under conditions of light and dark. Invest Ophthalmol Vis Sci. 1988 Jun;29(6):988–991. [PubMed] [Google Scholar]
- To K. W., Nadel A. J., Perlstein S. H., Margolis S. Bilateral optic disc neovascularization in association with retinitis pigmentosa. Can J Ophthalmol. 1991 Apr;26(3):152–155. [PubMed] [Google Scholar]
- Tzekov R., Arden G. B. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999 Jul-Aug;44(1):53–60. doi: 10.1016/s0039-6257(99)00063-6. [DOI] [PubMed] [Google Scholar]
- Uliss A. E., Gregor Z. J., Bird A. C. Retinitis pigmentosa and retinal neovascularization. Ophthalmology. 1986 Dec;93(12):1599–1603. doi: 10.1016/s0161-6420(86)33539-5. [DOI] [PubMed] [Google Scholar]
- Wang M. X., Sando R. S., Jr, Crandall A. S., Donoso L. A. Recent advances in the molecular genetics of retinitis pigmentosa. Curr Opin Ophthalmol. 1995 Jun;6(3):1–7. doi: 10.1097/00055735-199506000-00001. [DOI] [PubMed] [Google Scholar]
- Wetzig P. C., Jepson C. N. Treatment of diabetic retinopathy by light coagulation. Am J Ophthalmol. 1966 Sep;62(3):459–465. doi: 10.1016/0002-9394(66)91325-0. [DOI] [PubMed] [Google Scholar]
- Williams B. A potential role for angiotensin II-induced vascular endothelial growth factor expression in the pathogenesis of diabetic nephropathy? Miner Electrolyte Metab. 1998;24(6):400–405. doi: 10.1159/000057401. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B. Beta-cell mitochondria in the regulation of insulin secretion: a new culprit in type II diabetes. Diabetologia. 2000 Mar;43(3):265–277. doi: 10.1007/s001250050044. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Zito I., Thiselton D. L., Gorin M. B., Stout J. T., Plant C., Bird A. C., Bhattacharya S. S., Hardcastle A. J. Identification of novel RPGR (retinitis pigmentosa GTPase regulator) mutations in a subset of X-linked retinitis pigmentosa families segregating with the RP3 locus. Hum Genet. 1999 Jul-Aug;105(1-2):57–62. doi: 10.1007/s004399900110. [DOI] [PubMed] [Google Scholar]
- van Driel M. A., Maugeri A., Klevering B. J., Hoyng C. B., Cremers F. P. ABCR unites what ophthalmologists divide(s) Ophthalmic Genet. 1998 Sep;19(3):117–122. doi: 10.1076/opge.19.3.117.2187. [DOI] [PubMed] [Google Scholar]