Abstract
AIM—To examine the contribution of infiltrating cells in the local production of cytokines within the vitreous of patients with proliferative vitreoretinopathy (PVR). METHODS—The presence of mRNA coding for IL-6, IL-8, IL-1β, IL-1α, TNFα, IFNγ, IL-12, and HPRT was investigated in 25 vitreous samples from patients with PVR, 11 vitreous samples from patients with retinal detachment (RD) not complicated by PVR, and 10 vitreous samples from patients with macular hole (MH). A quantitative reverse transcriptase polymerase chain reaction (RT-PCR) using an internal competitor was used to investigate these samples. From these samples, 15 PVR, 8 RD, and 8 MH were analysed for the protein levels of the same cytokines using enzyme linked immunosorbent assay (ELISA). Spearman correlation was used to test any association between mRNA and cytokine protein levels, as an indicator of the contribution these cells make to the intravitreal cytokine milieu. RESULTS—A strong correlation was found between mRNA and their respective cytokine levels (protein products) for IL-6, IL-8, IL-1β, IL-1α, TNFα, IFNγ (Spearman r = 0.83, 0.73, 0.67, 0.91, 0.73, and 0.73 respectively), but not for IL-12. The median levels of IL-6, IL-8, IL-1β, and IFNγ mRNA and their respective cytokines were significantly higher (p <0.05) in patients with PVR than in those with macular hole. There was no statistically significant difference in the median levels of IL-1α mRNA between PVR and MH but the cytokine IL-1α was detected at a significantly higher level in PVR compared with MH patients. Between PVR and RD patients, there was no statistically significant difference in mRNA levels for all the investigated cytokines (p >0.05) except for IL-6 where there was a statistical significance (p= 0.038). In contrast, the median levels of IL-6, IL-8, and IL-1β cytokines were significantly higher (p <0.05) in patients with PVR than in those with RD, whereas for IL-1α and IFNγ no significant statistical difference was detected between PVR and RD patients (p >0.05). When results of RD and MH patients were compared, a statistical difference was only detected in mRNA levels of INFγ (p = 0.008). However, no difference was detected for INFγ (protein product) or for any of the other cytokines between RD and MH patients. CONCLUSION—Levels of both protein and mRNA encoding IL-6, IL-8, IL-1β, and IFNγ is significantly increased in vitreous samples from patients with PVR. The strong correlation between ELISA detectable cytokines (protein products) and their respective mRNA levels suggest that intravitreal, invasive cells are the major source of these cytokines, with the exception of IL-12. Cells invading the vitreous do not appear to locally produce IL-12 mRNA. This would appear to implicate cells peripheral to the vitreal mass as the major source of this cytokine.
Full Text
The Full Text of this article is available as a PDF (250.2 KB).
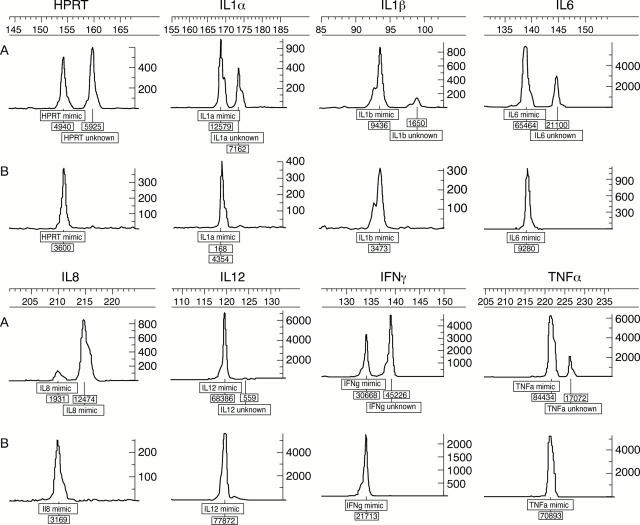
Figure 1 .
A typical electropherogram plot showing the size and area peaks for mimic and unknown (sample) of investigated cytokines. (A) Positive samples, (B) negative samples. Unknown concentration = (area of unknown/area of mimic) × mimic concentration.
Figure 2 .
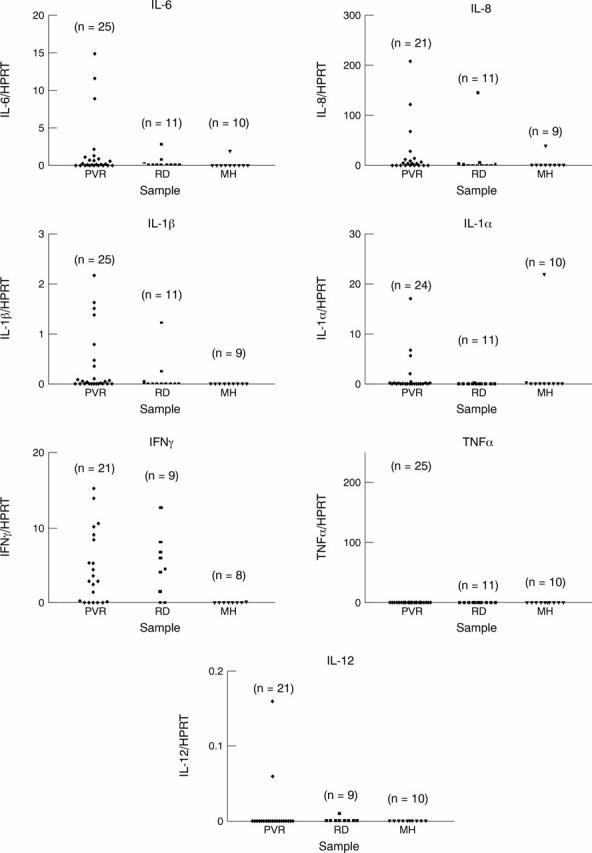
mRNA levels of different cytokines normalised to HPRT in vitreous aspirates obtained from patients with proliferative vitreoretinopathy (PVR), retinal detachment (RD), and macular hole (MH) (control). n = number of investigated samples.
Figure 3 .
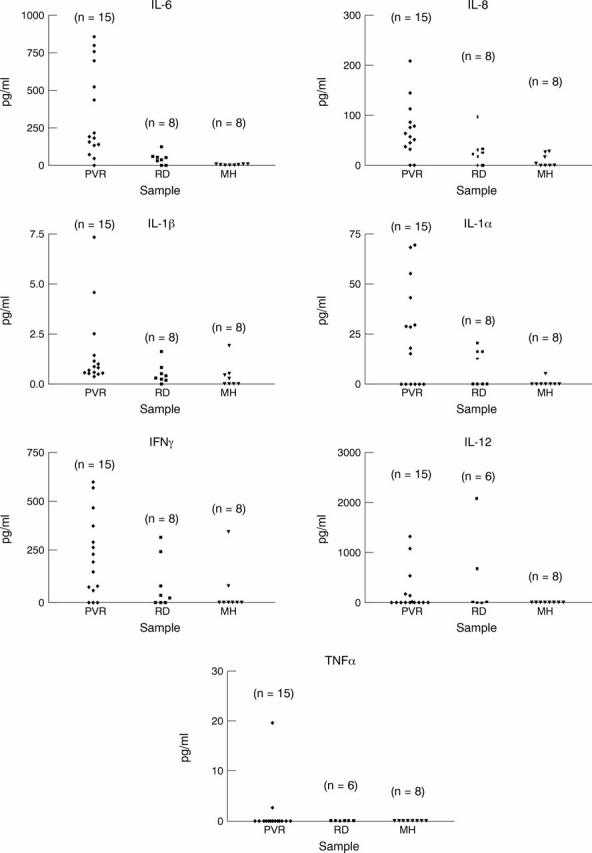
Levels of different cytokines in vitreous aspirates obtained from patients with proliferative vitreoretinopathy (PVR), retinal detachment (RD), and macular hole (MH) (control). n = number of investigated samples. Detection levels were (0.3, 1.6, 0.125, 3.9, 0.7, 15.6, and 0.7 pg/ml for IL-6, IL-8, IL-1β, IL-1α, TNFα, IFNγ, and IL-12 respectively.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu el-Asrar A. M., Van Damme J., Put W., Veckeneer M., Dralands L., Billiau A., Missotten L. Monocyte chemotactic protein-1 in proliferative vitreoretinal disorders. Am J Ophthalmol. 1997 May;123(5):599–606. doi: 10.1016/s0002-9394(14)71072-4. [DOI] [PubMed] [Google Scholar]
- Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990 Aug;4(11):2860–2867. [PubMed] [Google Scholar]
- Aksünger A., Or M., Okur H., Hasanreisoğlu B., Akbatur H. Role of interleukin 8 in the pathogenesis of proliferative vitreoretinopathy. Ophthalmologica. 1997;211(4):223–225. doi: 10.1159/000310794. [DOI] [PubMed] [Google Scholar]
- Balkwill F. R., Burke F. The cytokine network. Immunol Today. 1989 Sep;10(9):299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- Baudouin C., Fredj-Reygrobellet D., Gordon W. C., Baudouin F., Peyman G., Lapalus P., Gastaud P., Bazan N. G. Immunohistologic study of epiretinal membranes in proliferative vitreoretinopathy. Am J Ophthalmol. 1990 Dec 15;110(6):593–598. doi: 10.1016/s0002-9394(14)77054-0. [DOI] [PubMed] [Google Scholar]
- Campochiaro P. A. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol. 1997 Feb;115(2):237–241. doi: 10.1001/archopht.1997.01100150239014. [DOI] [PubMed] [Google Scholar]
- Camussi G., Albano E., Tetta C., Bussolino F. The molecular action of tumor necrosis factor-alpha. Eur J Biochem. 1991 Nov 15;202(1):3–14. doi: 10.1111/j.1432-1033.1991.tb16337.x. [DOI] [PubMed] [Google Scholar]
- Cavaillon J. M., Haeffner-Cavaillon N. Cytokines et inflammation. Rev Prat. 1993 Mar 1;43(5):547–552. [PubMed] [Google Scholar]
- Charteris D. G., Hiscott P., Grierson I., Lightman S. L. Proliferative vitreoretinopathy. Lymphocytes in epiretinal membranes. Ophthalmology. 1992 Sep;99(9):1364–1367. doi: 10.1016/s0161-6420(92)31793-2. [DOI] [PubMed] [Google Scholar]
- Charteris D. G. Proliferative vitreoretinopathy: pathobiology, surgical management, and adjunctive treatment. Br J Ophthalmol. 1995 Oct;79(10):953–960. doi: 10.1136/bjo.79.10.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaum E. Proliferative vitreoretinopathy. Int Ophthalmol Clin. 1995 Winter;35(1):163–173. doi: 10.1097/00004397-199503510-00017. [DOI] [PubMed] [Google Scholar]
- Choudhury P., Chen W., Hunt R. C. Production of platelet-derived growth factor by interleukin-1 beta and transforming growth factor-beta-stimulated retinal pigment epithelial cells leads to contraction of collagen gels. Invest Ophthalmol Vis Sci. 1997 Apr;38(5):824–833. [PubMed] [Google Scholar]
- Clementi M., Menzo S., Bagnarelli P., Manzin A., Valenza A., Varaldo P. E. Quantitative PCR and RT-PCR in virology. PCR Methods Appl. 1993 Feb;2(3):191–196. doi: 10.1101/gr.2.3.191. [DOI] [PubMed] [Google Scholar]
- D'Andrea A., Rengaraju M., Valiante N. M., Chehimi J., Kubin M., Aste M., Chan S. H., Kobayashi M., Young D., Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992 Nov 1;176(5):1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean G. A., Higgins J., LaVoy A., Fan Z., Pedersen N. C. Measurement of feline cytokine gene expression by quantitative-competitive RT-PCR. Vet Immunol Immunopathol. 1998 May 15;63(1-2):73–82. doi: 10.1016/s0165-2427(98)00084-1. [DOI] [PubMed] [Google Scholar]
- Diviacco S., Norio P., Zentilin L., Menzo S., Clementi M., Biamonti G., Riva S., Falaschi A., Giacca M. A novel procedure for quantitative polymerase chain reaction by coamplification of competitive templates. Gene. 1992 Dec 15;122(2):313–320. doi: 10.1016/0378-1119(92)90220-j. [DOI] [PubMed] [Google Scholar]
- El-Ghrably I. A., Dua H. S., Orr G. M., Fischer D., Tighe P. J. Detection of cytokine mRNA production in infiltrating cells in proliferative vitreoretinopathy using reverse transcription polymerase chain reaction. Br J Ophthalmol. 1999 Nov;83(11):1296–1299. doi: 10.1136/bjo.83.11.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elner S. G., Elner V. M., Jaffe G. J., Stuart A., Kunkel S. L., Strieter R. M. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995 Nov;14(11):1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- Elner V. M., Burnstine M. A., Strieter R. M., Kunkel S. L., Elner S. G. Cell-associated human retinal pigment epithelium interleukin-8 and monocyte chemotactic protein-1: immunochemical and in-situ hybridization analyses. Exp Eye Res. 1997 Dec;65(6):781–789. doi: 10.1006/exer.1997.0380. [DOI] [PubMed] [Google Scholar]
- Elner V. M., Strieter R. M., Elner S. G., Baggiolini M., Lindley I., Kunkel S. L. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990 Apr;136(4):745–750. [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Chantry D., Haworth C., Turner M., Abney E., Buchan G., Barrett K., Barkley D., Chu A. Cytokine production in the rheumatoid joint: implications for treatment. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):480–486. [PubMed] [Google Scholar]
- Glaser B. M., Cardin A., Biscoe B. Proliferative vitreoretinopathy. The mechanism of development of vitreoretinal traction. Ophthalmology. 1987 Apr;94(4):327–332. doi: 10.1016/s0161-6420(87)33443-8. [DOI] [PubMed] [Google Scholar]
- Hitchins C. A., Grierson I. Intravitreal injection of fibroblasts: the pathological effects on the ocular tissues of the rabbit following an intravitreal injection of autologous skin fibroblasts. Br J Ophthalmol. 1988 Jul;72(7):498–510. doi: 10.1136/bjo.72.7.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe G. J., Roberts W. L., Wong H. L., Yurochko A. D., Cianciolo G. J. Monocyte-induced cytokine expression in cultured human retinal pigment epithelial cells. Exp Eye Res. 1995 May;60(5):533–543. doi: 10.1016/s0014-4835(05)80068-5. [DOI] [PubMed] [Google Scholar]
- Jerdan J. A., Pepose J. S., Michels R. G., Hayashi H., de Bustros S., Sebag M., Glaser B. M. Proliferative vitreoretinopathy membranes. An immunohistochemical study. Ophthalmology. 1989 Jun;96(6):801–810. doi: 10.1016/s0161-6420(89)32818-1. [DOI] [PubMed] [Google Scholar]
- Kauffmann D. J., van Meurs J. C., Mertens D. A., Peperkamp E., Master C., Gerritsen M. E. Cytokines in vitreous humor: interleukin-6 is elevated in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):900–906. [PubMed] [Google Scholar]
- Kenarova B., Voinov L., Apostolov C., Vladimirova R., Misheva A. Levels of some cytokines in subretinal fluid in proliferative vitreoretinopathy and rhegmatogenous retinal detachment. Eur J Ophthalmol. 1997 Jan-Mar;7(1):64–67. doi: 10.1177/112067219700700112. [DOI] [PubMed] [Google Scholar]
- Kon C. H., Occleston N. L., Aylward G. W., Khaw P. T. Expression of vitreous cytokines in proliferative vitreoretinopathy: a prospective study. Invest Ophthalmol Vis Sci. 1999 Mar;40(3):705–712. [PubMed] [Google Scholar]
- Kontakou M., Przemioslo R. T., Sturgess R. P., Limb A. G., Ciclitira P. J. Expression of tumour necrosis factor-alpha, interleukin-6, and interleukin-2 mRNA in the jejunum of patients with coeliac disease. Scand J Gastroenterol. 1995 May;30(5):456–463. doi: 10.3109/00365529509093307. [DOI] [PubMed] [Google Scholar]
- Kuppner M. C., McKillop-Smith S., Forrester J. V. TGF-beta and IL-1 beta act in synergy to enhance IL-6 and IL-8 mRNA levels and IL-6 production by human retinal pigment epithelial cells. Immunology. 1995 Feb;84(2):265–271. [PMC free article] [PubMed] [Google Scholar]
- Limb G. A., Alam A., Earley O., Green W., Chignell A. H., Dumonde D. C. Distribution of cytokine proteins within epiretinal membranes in proliferative vitreoretinopathy. Curr Eye Res. 1994 Nov;13(11):791–798. doi: 10.3109/02713689409025133. [DOI] [PubMed] [Google Scholar]
- Limb G. A., Earley O., Jones S. E., LeRoy F., Chignell A. H., Dumonde D. C. Expression of mRNA coding for TNF alpha, IL-1 beta and IL-6 by cells infiltrating retinal membranes. Graefes Arch Clin Exp Ophthalmol. 1994 Nov;232(11):646–651. doi: 10.1007/BF00171378. [DOI] [PubMed] [Google Scholar]
- Limb G. A., Little B. C., Meager A., Ogilvie J. A., Wolstencroft R. A., Franks W. A., Chignell A. H., Dumonde D. C. Cytokines in proliferative vitreoretinopathy. Eye (Lond) 1991;5(Pt 6):686–693. doi: 10.1038/eye.1991.126. [DOI] [PubMed] [Google Scholar]
- McMenamin P. G. The distribution of immune cells in the uveal tract of the normal eye. Eye (Lond) 1997;11(Pt 2):183–193. doi: 10.1038/eye.1997.49. [DOI] [PubMed] [Google Scholar]
- Nagasaki H., Shinagawa K. Risk factors for proliferative vitreoretinopathy. Curr Opin Ophthalmol. 1995 Jun;6(3):70–75. doi: 10.1097/00055735-199506000-00012. [DOI] [PubMed] [Google Scholar]
- Pannetier C., Delassus S., Darche S., Saucier C., Kourilsky P. Quantitative titration of nucleic acids by enzymatic amplification reactions run to saturation. Nucleic Acids Res. 1993 Feb 11;21(3):577–583. doi: 10.1093/nar/21.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor J. C. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998 Jul-Aug;43(1):3–18. doi: 10.1016/s0039-6257(98)00023-x. [DOI] [PubMed] [Google Scholar]
- Planck S. R., Andresevic J., Chen J. C., Holmes D. L., Rodden W., Westra I., Wu S. C., Huang X. N., Kay G., Wilson D. J. Expression of growth factor mRNA in rabbit PVR model systems. Curr Eye Res. 1992 Nov;11(11):1031–1039. doi: 10.3109/02713689209015074. [DOI] [PubMed] [Google Scholar]
- Planck S. R., Huang X. N., Robertson J. E., Rosenbaum J. T. Retinal pigment epithelial cells produce interleukin-1 beta and granulocyte-macrophage colony-stimulating factor in response to interleukin-1 alpha. Curr Eye Res. 1993 Mar;12(3):205–212. doi: 10.3109/02713689308999465. [DOI] [PubMed] [Google Scholar]
- Roberge F. G., Caspi R. R., Nussenblatt R. B. Glial retinal Müller cells produce IL-1 activity and have a dual effect on autoimmune T helper lymphocytes. Antigen presentation manifested after removal of suppressive activity. J Immunol. 1988 Apr 1;140(7):2193–2196. [PubMed] [Google Scholar]
- Sutterwala F. S., Mosser D. M. The taming of IL-12: suppressing the production of proinflammatory cytokines. J Leukoc Biol. 1999 May;65(5):543–551. [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]