Abstract
AIMS—Amniotic membrane (AM) transplantation reduces inflammation in a variety of ocular surface disorders. The aim of this study was to determine if AM stroma suppresses the expression of the IL-1 gene family in cultured human corneal limbal epithelial cells. METHODS—Human corneal limbal epithelial cells were cultured from limbocorneal explants of donor eyes on plastic or on the AM stroma. Transcript expression of IL-1α, IL-1β, IL-1 receptor antagonist (RA), and GAPDH was compared with or without addition of lipopolysaccharide to their serum-free media for 24 hours using RNAse protection assay (RPA). Their protein production in the supernatant was analysed by ELISA. RESULTS—Expression of IL-1α and IL-1β transcripts and proteins was significantly reduced by cells cultured on the AM stromal matrix compared with plastic cultures whether lipopolysaccharide was added or not. Moreover, expression of IL-1 RA by cells cultured in the lipopolysaccharide-free medium was upregulated by AM stromal matrix. The ratio between IL-1 RA and IL-1α protein levels in AM cultures was higher than in plastic cultures. CONCLUSIONS—AM stromal matrix markedly suppresses lipopolysaccharide induced upregulation of both IL-1α and IL-1β. These data may explain in part the effect of AM transplantation in reducing ocular surface inflammation, underscoring the unique feature of the AM as a substrate for tissue engineering.
Full Text
The Full Text of this article is available as a PDF (168.0 KB).
Figure 1 .
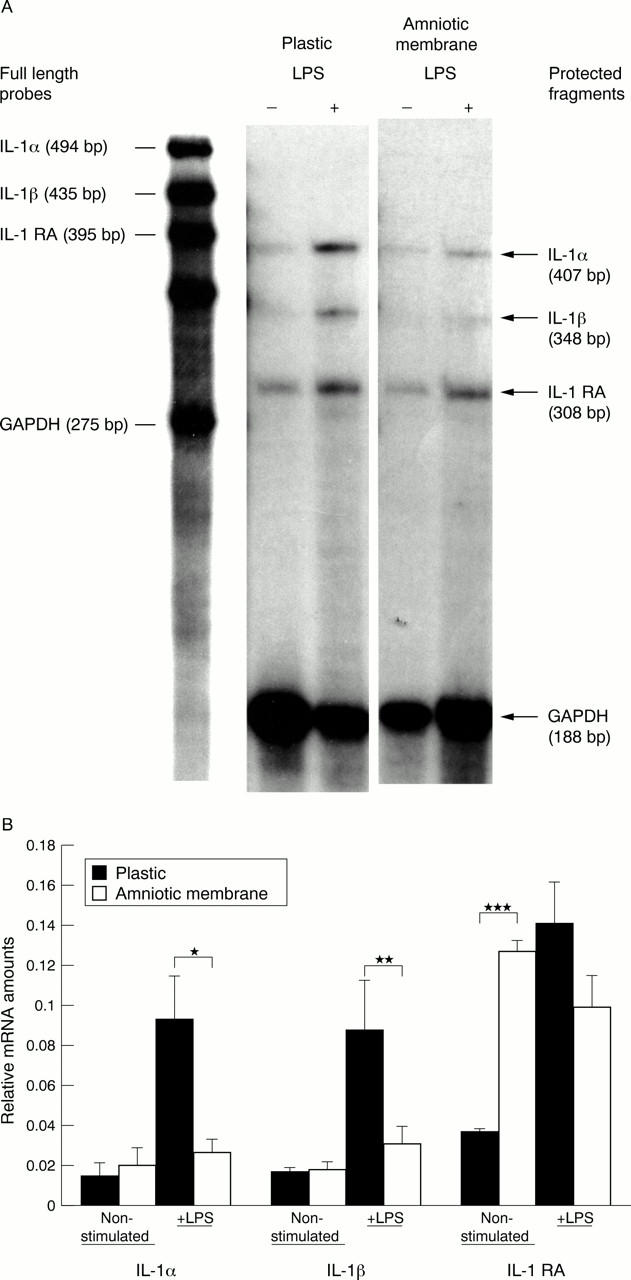
(A) Ribonuclease protection assay of RNAs extracted from cultured human corneal epithelial cells. Cells were switched to a serum-free medium with or without 10 µg/ml LPS. Samples were hybridised with riboprobes to IL-1α, IL-1β, IL-1 RA, and GAPDH, followed by treatment with ribonuclease and inactivation with proteinase K. Protected RNA fragments were precipitated and separated on a 6% polyacrylamide Urea-TBE sequencing gel. Numbers in parentheses show the size in base pairs. (B) Graphical presentation of the relative amounts for IL-1α, IL-1β, and IL-1RA mRNAs when corrected for the different amounts of GAPDH mRNA in the same sample. A significant increase in the amount of IL-1α and IL-1β mRNAs was observed following the treatment with LPS in plastic cultures, whereas these amounts were significantly decreased in AM cultures for IL-1α (*p=0.029) and had a marginally significant decrease for IL-1β (**p=0.054). In contrast, the amount of IL-1RA mRNA was significantly upregulated in non-stimulated cells cultured on the AM (***p=0.0002). Data are expressed as the mean (SEM) from five different experiments based on primary cultures from five different donor corneas, respectively.
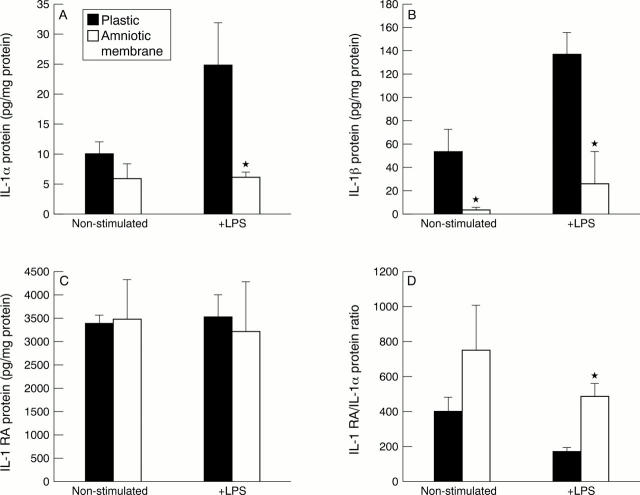
Figure 2 .
Protein amounts of IL-1α (A), IL-1β (B), and IL-1RA (C) measured by ELISA in conditioned media of human limbal epithelium cultured on plastic or AM, and collected after 24 hours in a serum-free medium with or without LPS. (A) A significant decrease of IL-1α protein was noted in LPS stimulated cells on AM (*p=0.009). (B) A significant decrease of IL-1β protein was noted in LPS stimulated and non-stimulated cells on AM (*p=0.017). (C) No change was noted in IL-1RA protein. (D) The IL-1RA/IL-1α ratio was increased in LPS stimulated cells on AM (*p=0.008). Data are expressed as the mean (SEM) from five independent experiments using five different donor corneas.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afonso A. A., Sobrin L., Monroy D. C., Selzer M., Lokeshwar B., Pflugfelder S. C. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999 Oct;40(11):2506–2512. [PubMed] [Google Scholar]
- Barton K., Monroy D. C., Nava A., Pflugfelder S. C. Inflammatory cytokines in the tears of patients with ocular rosacea. Ophthalmology. 1997 Nov;104(11):1868–1874. doi: 10.1016/s0161-6420(97)30014-1. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Weaver V. M., Lelièvre S. A., Wang F., Petersen O. W., Schmeichel K. L. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999 Apr 1;59(7 Suppl):1757–1764s. [PubMed] [Google Scholar]
- Chen H. J., Pires R. T., Tseng S. C. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000 Aug;84(8):826–833. doi: 10.1136/bjo.84.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Griffith M., Osborne R., Munger R., Xiong X., Doillon C. J., Laycock N. L., Hakim M., Song Y., Watsky M. A. Functional human corneal equivalents constructed from cell lines. Science. 1999 Dec 10;286(5447):2169–2172. doi: 10.1126/science.286.5447.2169. [DOI] [PubMed] [Google Scholar]
- He Y. G., Alizadeh H., Kinoshita K., McCulley J. P. Experimental transplantation of cultured human limbal and amniotic epithelial cells onto the corneal surface. Cornea. 1999 Sep;18(5):570–579. [PubMed] [Google Scholar]
- Jumblatt M. M., Neufeld A. H. Beta-adrenergic and serotonergic responsiveness of rabbit corneal epithelial cells in culture. Invest Ophthalmol Vis Sci. 1983 Aug;24(8):1139–1143. [PubMed] [Google Scholar]
- Kim J. C., Tseng S. C. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995 Sep;14(5):473–484. [PubMed] [Google Scholar]
- Kruse F. E., Rohrschneider K., Völcker H. E. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999 Aug;106(8):1504–1511. doi: 10.1016/S0161-6420(99)90444-X. [DOI] [PubMed] [Google Scholar]
- Lee S. B., Li D. Q., Tan D. T., Meller D. C., Tseng S. C. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000 Apr;20(4):325–334. [PubMed] [Google Scholar]
- Lee S. H., Tseng S. C. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997 Mar;123(3):303–312. doi: 10.1016/s0002-9394(14)70125-4. [DOI] [PubMed] [Google Scholar]
- Mast B. A., Haynes J. H., Krummel T. M., Diegelmann R. F., Cohen I. K. In vivo degradation of fetal wound hyaluronic acid results in increased fibroplasia, collagen deposition, and neovascularization. Plast Reconstr Surg. 1992 Mar;89(3):503–509. doi: 10.1097/00006534-199203000-00019. [DOI] [PubMed] [Google Scholar]
- Mathers W. D. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993 Mar;100(3):347–351. doi: 10.1016/s0161-6420(93)31643-x. [DOI] [PubMed] [Google Scholar]
- Meller D., Pires R. T., Mack R. J., Figueiredo F., Heiligenhaus A., Park W. C., Prabhasawat P., John T., McLeod S. D., Steuhl K. P. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000 May;107(5):980–990. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Neumann A., Schinzel R., Palm D., Riederer P., Münch G. High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-kappaB activation and cytokine expression. FEBS Lett. 1999 Jun 25;453(3):283–287. doi: 10.1016/s0014-5793(99)00731-0. [DOI] [PubMed] [Google Scholar]
- Park W. C., Tseng S. C. Modulation of acute inflammation and keratocyte death by suturing, blood, and amniotic membrane in PRK. Invest Ophthalmol Vis Sci. 2000 Sep;41(10):2906–2914. [PubMed] [Google Scholar]
- Pires R. T., Tseng S. C., Prabhasawat P., Puangsricharern V., Maskin S. L., Kim J. C., Tan D. T. Amniotic membrane transplantation for symptomatic bullous keratopathy. Arch Ophthalmol. 1999 Oct;117(10):1291–1297. doi: 10.1001/archopht.117.10.1291. [DOI] [PubMed] [Google Scholar]
- Prabhasawat P., Barton K., Burkett G., Tseng S. C. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997 Jun;104(6):974–985. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- Puangsricharern V., Tseng S. C. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995 Oct;102(10):1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- Roskelley C. D., Srebrow A., Bissell M. J. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995 Oct;7(5):736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A. I., Maier-Reif K., Graeve T. Constructing an in vitro cornea from cultures of the three specific corneal cell types. In Vitro Cell Dev Biol Anim. 1999 Oct;35(9):515–526. doi: 10.1007/s11626-999-0062-0. [DOI] [PubMed] [Google Scholar]
- Shimazaki J., Yang H. Y., Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997 Dec;104(12):2068–2076. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- Solomon A., Rosenblatt M., Li D. Q., Liu Z., Monroy D., Ji Z., Lokeshwar B. L., Pflugfelder S. C. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Invest Ophthalmol Vis Sci. 2000 Aug;41(9):2544–2557. [PubMed] [Google Scholar]
- Sunderland C. A., Bulmer J. N., Luscombe M., Redman C. W., Stirrat G. M. Immunohistological and biochemical evidence for a role for hyaluronic acid in the growth and development of the placenta. J Reprod Immunol. 1985 Nov;8(2-3):197–212. doi: 10.1016/0165-0378(85)90041-5. [DOI] [PubMed] [Google Scholar]
- Tsai R. J., Tseng S. C. Effect of stromal inflammation on the outcome of limbal transplantation for corneal surface reconstruction. Cornea. 1995 Sep;14(5):439–449. [PubMed] [Google Scholar]
- Tseng S. C., Hirst L. W., Maumenee A. E., Kenyon K. R., Sun T. T., Green W. R. Possible mechanisms for the loss of goblet cells in mucin-deficient disorders. Ophthalmology. 1984 Jun;91(6):545–552. doi: 10.1016/s0161-6420(84)34251-8. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Li D. Q., Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999 Jun;179(3):325–335. doi: 10.1002/(SICI)1097-4652(199906)179:3<325::AID-JCP10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Prabhasawat P., Barton K., Gray T., Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998 Apr;116(4):431–441. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Prabhasawat P., Lee S. H. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol. 1997 Dec;124(6):765–774. doi: 10.1016/s0002-9394(14)71693-9. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Satake Y., Ohyama M., Toda I., Takano Y., Ono M., Shinozaki N., Shimazaki J. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol. 1996 Jul;122(1):38–52. doi: 10.1016/s0002-9394(14)71962-2. [DOI] [PubMed] [Google Scholar]
- Weng J., Mohan R. R., Li Q., Wilson S. E. IL-1 upregulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells: interleukin-1 beta expression in the cornea. Cornea. 1997 Jul;16(4):465–471. [PubMed] [Google Scholar]
- Wilson S. E., Lloyd S. A., He Y. G. Glucocorticoid receptor and interleukin-1 receptor messenger RNA expression in corneal cells. Cornea. 1994 Jan;13(1):4–8. doi: 10.1097/00003226-199401000-00002. [DOI] [PubMed] [Google Scholar]