Abstract
AIMS—To evaluate whether the b-wave of the dark adapted flash electroretinogram (ERG) is affected by glaucomatous damage. METHODS—ERGs were recorded in 35 patients aged 33-65 years with advanced asymmetrical glaucomas (interocular difference of perimetric defects (mean deviation) >2 dB between the two fellow eyes of the glaucoma patients, primary and secondary open angle and low tension glaucomas) and 17 normal subjects matched for age and sex using white flashes of a xenon discharge tube in a Ganzfeld stimulator. After 30 minutes of dark adaptation luminance response functions were obtained using flashes of increasing scotopic luminance (highest 9.4 cd/s/m2, lowest 5.5 log units below it). The parameters Vmax, n, and K of the Naka-Rushton equation were computed from the measurement values based on the usual fitting procedure. These parameters, together with b-wave amplitudes and implicit times for all flash intensities, were compared interocularly and between the normal subjects and those with glaucoma. Correlations were computed between interocular differences of the mean deviation and interocular differences of Vmax, n, K, b-wave amplitudes, and implicit times between the two fellow eyes of the patients with asymmetrical glaucomatous damage. RESULTS—Implicit times were significantly longer (p<0.005) in the glaucoma patients than in the normal group for flash intensities of 9.4, 5.3, 1.7, 0.53, and 0.17 cd/s/m2. b-Wave amplitudes did not differ significantly between the two study groups. Comparing the two fellow eyes of each patient with glaucoma, Vmax was significantly higher in the less damaged eye than in the more damaged eye. The interocular differences in the mean deviation correlated significantly with the interocular differences in the b-wave amplitudes, implicit times, and Vmax. CONCLUSIONS—These results suggest that glaucomas can lead to electrophysiologically measurable damage of the inner nuclear layer.
Full Text
The Full Text of this article is available as a PDF (202.4 KB).
Figure 1 .
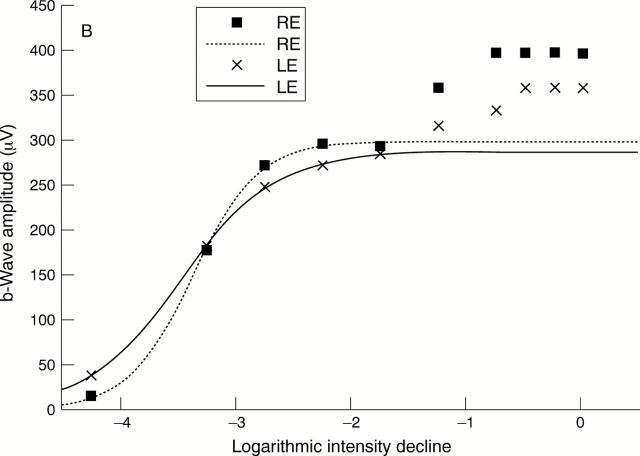
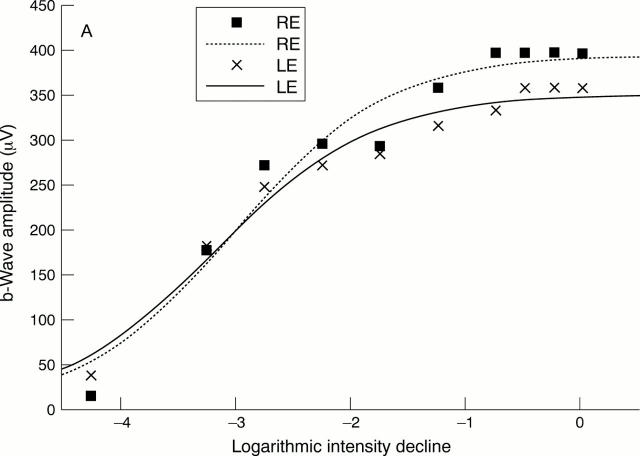
(A) Comparison between the intensity response data and the first model of the Naka-Rushton function fitted to all data points. The patient suffered from asymmetrical juvenile open angle glaucoma of the left eye (mean deviation in right eye (RE) -1.0 dB, mean deviation in left eye (LE) 8.7 dB). The parameters of the Naka-Rushton equation are: Vmax = 395 µm, K = -3.02 log units, n = 0.65 for the right eye and Vmax = 351 µm, K = -3.19 log units, n = 0.63 for the left eye. (B) Intensity response data of the right and left eyes of the same patient. In contrast to (A), this figure demonstrates the second model of the Naka-Rushton equation with fit of the function to data points below -1.25 log units (0.53 cd/s/m2) and thus below the "second limb" of the intensity response function. The parameters of the second model of the Naka-Rushton equation are: Vmax = 298 µm, K = -3.36 log units, n = 1.55 for the right eye and Vmax = 288 µm, K = -3.47 log units, n = 1.06 for the left eye.
Figure 2 .
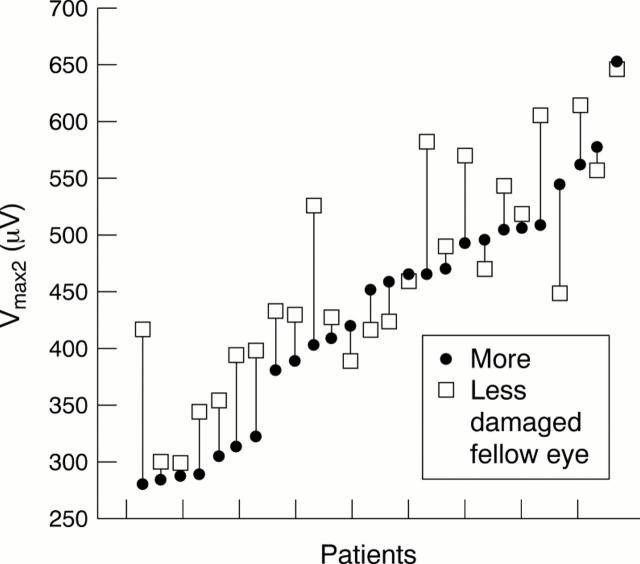
Vmax2 of the more and less damaged fellow eyes of all glaucoma patients included in the interocular comparison (interocular difference in mean deviation >2 dB). In 18 of the 26 patients Vmax2 was higher in the fellow eye with the less advanced glaucomatous damage.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birch D. G., Fish G. E. Rod ERGs in retinitis pigmentosa and cone-rod degeneration. Invest Ophthalmol Vis Sci. 1987 Jan;28(1):140–150. [PubMed] [Google Scholar]
- Carter-Dawson L., Shen F., Harwerth R. S., Smith E. L., 3rd, Crawford M. L., Chuang A. Glutamine immunoreactivity in Müller cells of monkey eyes with experimental glaucoma. Exp Eye Res. 1998 May;66(5):537–545. doi: 10.1006/exer.1997.0447. [DOI] [PubMed] [Google Scholar]
- FRANCOIS J. L'electroretinographie dans le glaucome. Acta Ophthalmol (Copenh) 1953;31(3):205–218. doi: 10.1111/j.1755-3768.1953.tb03286.x. [DOI] [PubMed] [Google Scholar]
- Fazio D. T., Heckenlively J. R., Martin D. A., Christensen R. E. The electroretinogram in advanced open-angle glaucoma. Doc Ophthalmol. 1986 Jun 16;63(1):45–54. doi: 10.1007/BF00153011. [DOI] [PubMed] [Google Scholar]
- Flammer J., Drance S. M., Augustiny L., Funkhouser A. Quantification of glaucomatous visual field defects with automated perimetry. Invest Ophthalmol Vis Sci. 1985 Feb;26(2):176–181. [PubMed] [Google Scholar]
- Hood D. C., Birch D. G. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci. 1992 Feb;8(2):107–126. doi: 10.1017/s0952523800009275. [DOI] [PubMed] [Google Scholar]
- Janssen P., Naskar R., Moore S., Thanos S., Thiel H. J. Evidence for glaucoma-induced horizontal cell alterations in the human retina. Ger J Ophthalmol. 1996 Nov;5(6):378–385. [PubMed] [Google Scholar]
- Kendell K. R., Quigley H. A., Kerrigan L. A., Pease M. E., Quigley E. N. Primary open-angle glaucoma is not associated with photoreceptor loss. Invest Ophthalmol Vis Sci. 1995 Jan;36(1):200–205. [PubMed] [Google Scholar]
- Knapp A. G., Schiller P. H. The contribution of on-bipolar cells to the electroretinogram of rabbits and monkeys. A study using 2-amino-4-phosphonobutyrate (APB). Vision Res. 1984;24(12):1841–1846. doi: 10.1016/0042-6989(84)90016-6. [DOI] [PubMed] [Google Scholar]
- Korth M., Nguyen N. X., Horn F., Martus P. Scotopic threshold response and scotopic PII in glaucoma. Invest Ophthalmol Vis Sci. 1994 Feb;35(2):619–625. [PubMed] [Google Scholar]
- LEYDHECKER G. The electroretinogram in glaucomatous eyes. Br J Ophthalmol. 1950 Sep;34(9):550–554. doi: 10.1136/bjo.34.9.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S., Jonas J. B. Decreased photoreceptor count in human eyes with secondary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 1992 Jul;33(8):2532–2536. [PubMed] [Google Scholar]
- Peachey N. S., Alexander K. R., Fishman G. A. The luminance-response function of the dark-adapted human electroretinogram. Vision Res. 1989;29(3):263–270. doi: 10.1016/0042-6989(89)90075-8. [DOI] [PubMed] [Google Scholar]
- Roecker E. B., Pulos E., Bresnick G. H., Severns M. Characterization of the electroretinographic scotopic B-wave amplitude in diabetic and normal subjects. Invest Ophthalmol Vis Sci. 1992 Apr;33(5):1575–1583. [PubMed] [Google Scholar]
- Shiells R. A., Falk G. Contribution of rod, on-bipolar, and horizontal cell light responses to the ERG of dogfish retina. Vis Neurosci. 1999 May-Jun;16(3):503–511. doi: 10.1017/s0952523899163119. [DOI] [PubMed] [Google Scholar]
- Standard for clinical electroretinography. International Standardization Committee. Arch Ophthalmol. 1989 Jun;107(6):816–819. doi: 10.1001/archopht.1989.01070010838024. [DOI] [PubMed] [Google Scholar]
- Tanihara H., Hangai M., Sawaguchi S., Abe H., Kageyama M., Nakazawa F., Shirasawa E., Honda Y. Up-regulation of glial fibrillary acidic protein in the retina of primate eyes with experimental glaucoma. Arch Ophthalmol. 1997 Jun;115(6):752–756. doi: 10.1001/archopht.1997.01100150754011. [DOI] [PubMed] [Google Scholar]
- Vaegan, Graham S. L., Goldberg I., Buckland L., Hollows F. C. Flash and pattern electroretinogram changes with optic atrophy and glaucoma. Exp Eye Res. 1995 Jun;60(6):697–706. doi: 10.1016/s0014-4835(05)80011-9. [DOI] [PubMed] [Google Scholar]