Abstract
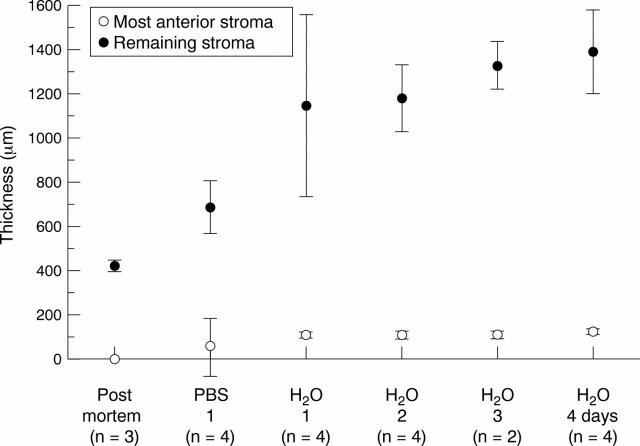
AIM—To analyse the human corneal stroma in extreme hydration to discover if its structure is responsible for corneal stability. METHODS—Corneas in several hydration states were used: postmortem control corneas (PM; n=3), corneas left for 1 day in phosphate buffered saline (PBS; n=4), and corneas left for 1 day (n=4), 2 days (n=4), 3 days (n=2), and 4 days (n=4) in deionised water. All corneas were fixed under standardised conditions and processed for light and electron microscopy. In addition, two fresh corneas from the operating theatre were studied which were processed 6 months after storage in sodium cacodylate buffer. RESULTS—After 1 day in deionised water maximal stromal swelling was reached which did not change up to 4 days. The stroma of deionised water corneas (1400 µm) was much thicker than that of PBS corneas (650 µm) and PM corneas (450 µm). Deionised water treatment led to disappearance of all keratocytes leaving only remnants of nuclei and large interlamellar spaces. In these specimens the distance between the collagen fibres had increased significantly, but the diameter of the collagen fibres did not seem to be affected. A remarkable observation was that the most anterior part of the stroma (100-120 µm) in all deionised water specimens and those stored for 6 months in buffer was not swollen, indicating that the tightly interwoven anterior lamellae are resistant to extreme non-physiological hydration states. CONCLUSIONS—The rigidity of the most anterior part of the corneal stroma in extreme hydration states points to an important role in maintenance of corneal curvature. Since a large part of this rigid anterior part of the stroma is either removed (PRK) or intersected (LASIK), it is possible that in the long run patients who underwent refractive surgery may be confronted with optical problems.
Full Text
The Full Text of this article is available as a PDF (306.7 KB).
Figure 1 .
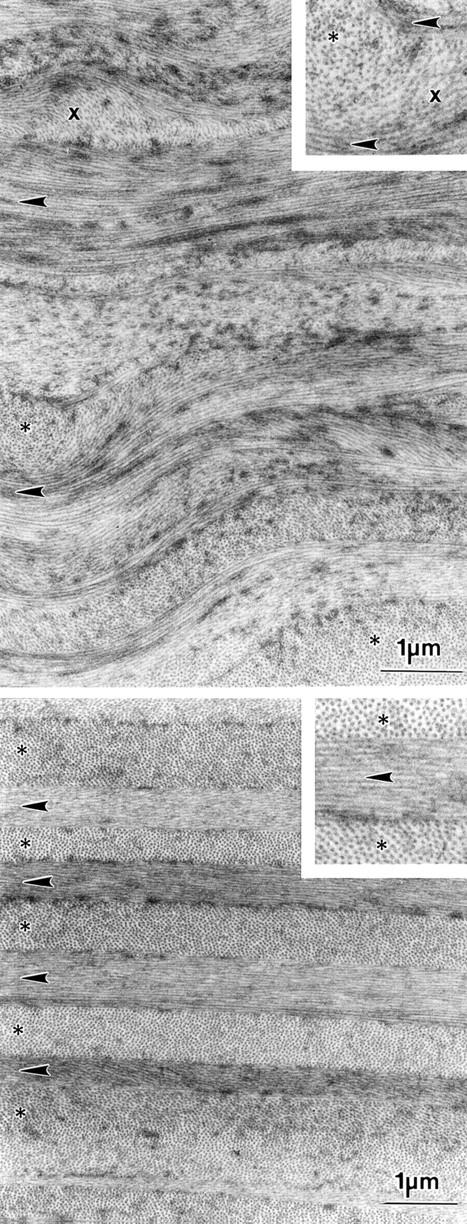
(Top) Electron micrograph of a cross section through the anterior stroma of the human cornea. Collagen lamellae are alternated and undulated. The different collagen bundles cross sectioned (*), longitudinally sectioned (arrowhead), and obliquely sectioned (x) alternate at irregular distances. (Bottom) Electron micrograph of the posterior stroma shows strictly alternating cross sectioned (*) and longitudinal sectioned (arrowhead) collagen bundles at more or less regular distances.
Figure 2 .
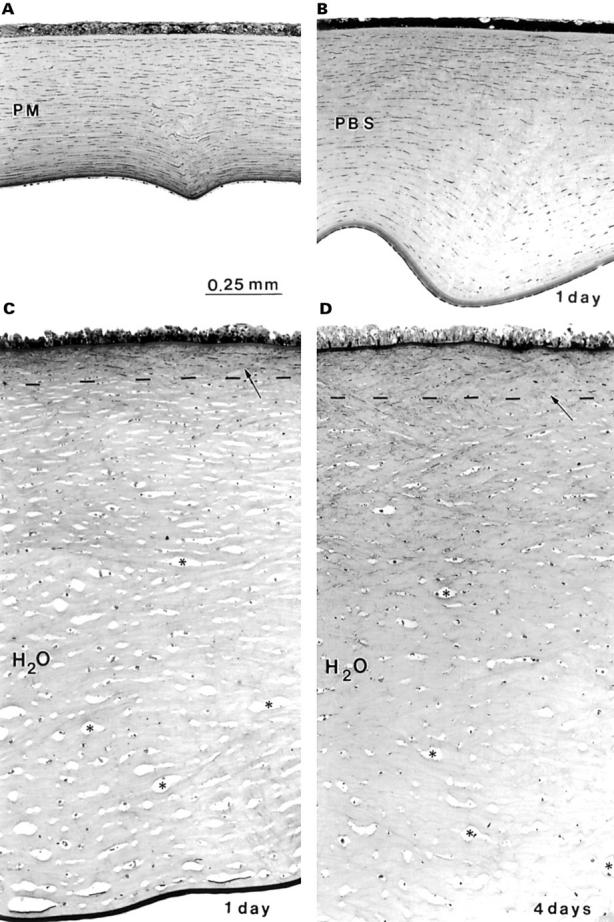
Light micrographs of semithin sections from corneas at different states of hydration. (A) Post mortem (PM); (B) phosphate buffered saline (PBS); (C) 1 day deionised water, and (D) 4 days deionised water. The magnification for all micrographs is the same. The most anterior stroma, above the broken line, is not affected by hydration (C and D, arrows). Most keratocytes in the mid and posterior stroma are shrunken or have disappeared and large interlaminar spaces (*) are left.
Figure 3 .
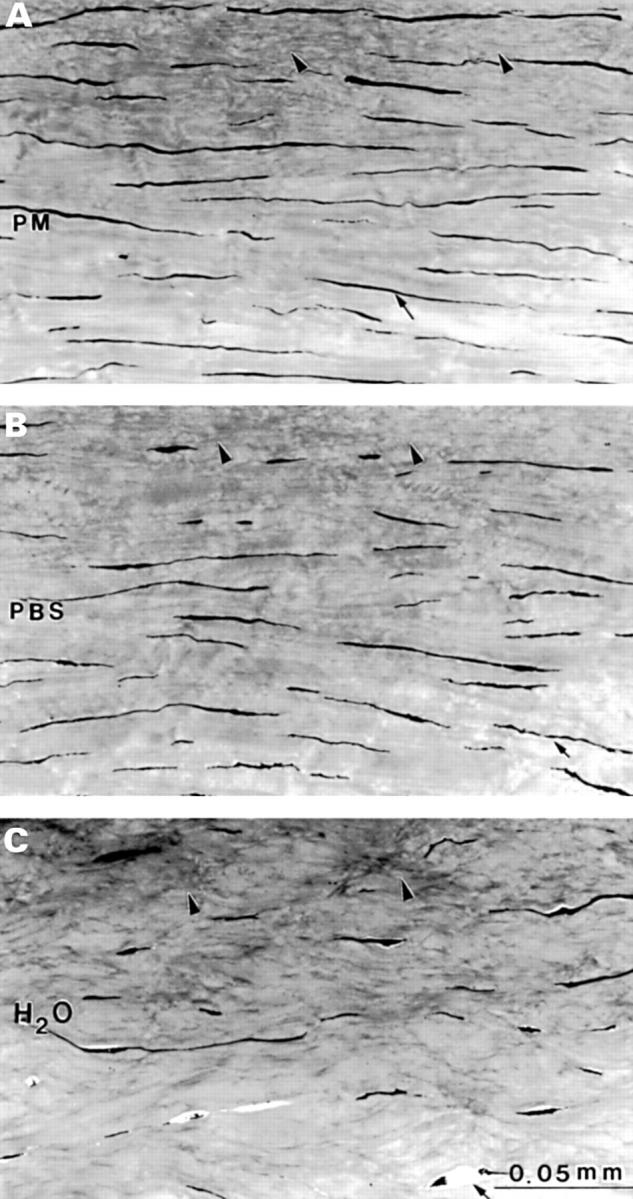
Light micrographs of the most anterior stroma in (A) post mortem (PM); (B) phosphate buffered saline (PBS), and (C) deionised water (H2O). In (A) and (B) more keratocyte profiles are present (arrows) than in (C). The undulated collagen bundles (arrowheads) in (A) and (B) are similar; (C) is different and characterised by irregularly arranged grey structures.
Figure 4 .
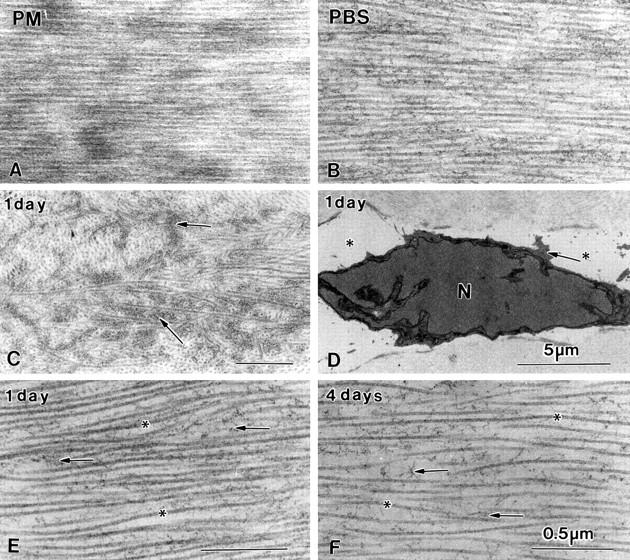
Longitudinal collagen fibres of a PM cornea (A) and a PBS cornea (B). The irregular grey structures in Figure 3C after 1 day in deionised water represent amorphous extracellular matrix (C, arrows). Only occasionally keratocyte nuclei with a small rim of cytoplasm are observed in mid stroma. Treatment with deionised water does not allow well stained cross sectioned collagen fibres in the posterior stroma. Longitudinal collagen fibres (*) surrounded by amorphous matrix (arrow) can be visualised (E and F). All bars represent 0.5 µm.
Figure 5 .
Thickness of the corneal stroma after treatment with deionised water for 1, 2, 3 and 4 days. These groups are compared with PM and PBS treated tissue.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alió J. L., Artola A., Claramonte P. J., Ayala M. J., Sánchez S. P. Complications of photorefractive keratectomy for myopia: two year follow-up of 3000 cases. J Cataract Refract Surg. 1998 May;24(5):619–626. doi: 10.1016/s0886-3350(98)80256-3. [DOI] [PubMed] [Google Scholar]
- Barker N. H., Couper T. A., Taylor H. R. Changes in corneal topography after laser in situ keratomileusis for myopia. J Refract Surg. 1999 Jan-Feb;15(1):46–52. doi: 10.3928/1081-597X-19990101-08. [DOI] [PubMed] [Google Scholar]
- Bettelheim F. A., Plessy B. The hydration of proteoglycans of bovine cornea. Biochim Biophys Acta. 1975 Jan 13;381(1):203–214. doi: 10.1016/0304-4165(75)90202-0. [DOI] [PubMed] [Google Scholar]
- Buratto L., Ferrari M. Indications, techniques, results, limits, and complications of laser in situ keratomileusis. Curr Opin Ophthalmol. 1997 Aug;8(4):59–66. doi: 10.1097/00055735-199708000-00009. [DOI] [PubMed] [Google Scholar]
- Castoro J. A., Bettelheim A. A., Bettelheim F. A. Water gradients across bovine cornea. Invest Ophthalmol Vis Sci. 1988 Jun;29(6):963–968. [PubMed] [Google Scholar]
- Corbett M. C., O'Brart D. P., Warburton F. G., Marshall J. Biologic and environmental risk factors for regression after photorefractive keratectomy. Ophthalmology. 1996 Sep;103(9):1381–1391. doi: 10.1016/s0161-6420(96)30494-6. [DOI] [PubMed] [Google Scholar]
- Corbett M. C., Prydal J. I., Verma S., Oliver K. M., Pande M., Marshall J. An in vivo investigation of the structures responsible for corneal haze after photorefractive keratectomy and their effect on visual function. Ophthalmology. 1996 Sep;103(9):1366–1380. doi: 10.1016/s0161-6420(96)30495-8. [DOI] [PubMed] [Google Scholar]
- Cristol S. M., Edelhauser H. F., Lynn M. J. A comparison of corneal stromal edema induced from the anterior or the posterior surface. Refract Corneal Surg. 1992 May-Jun;8(3):224–229. [PubMed] [Google Scholar]
- Daxer A., Fratzl P. Collagen fibril orientation in the human corneal stroma and its implication in keratoconus. Invest Ophthalmol Vis Sci. 1997 Jan;38(1):121–129. [PubMed] [Google Scholar]
- Farrell R. A., McCally R. L., Tatham P. E. Wave-length dependencies of light scattering in normal and cold swollen rabbit corneas and their structural implications. J Physiol. 1973 Sep;233(3):589–612. doi: 10.1113/jphysiol.1973.sp010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratzl P., Daxer A. Structural transformation of collagen fibrils in corneal stroma during drying. An x-ray scattering study. Biophys J. 1993 Apr;64(4):1210–1214. doi: 10.1016/S0006-3495(93)81487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund D. E., McCally R. L., Farrell R. A., Cristol S. M., L'Hernault N. L., Edelhauser H. F. Ultrastructure in anterior and posterior stroma of perfused human and rabbit corneas. Relation to transparency. Invest Ophthalmol Vis Sci. 1995 Jul;36(8):1508–1523. [PubMed] [Google Scholar]
- Gallagher B., Maurice D. Striations of light scattering in the corneal stroma. J Ultrastruct Res. 1977 Oct;61(1):100–114. doi: 10.1016/s0022-5320(77)90009-0. [DOI] [PubMed] [Google Scholar]
- Hara T., Maurice D. M. Changes in the swelling pressure of the corneal stroma with time, hydration and temperature, determined by a new method. Exp Eye Res. 1972 Jul;14(1):40–48. doi: 10.1016/0014-4835(72)90140-6. [DOI] [PubMed] [Google Scholar]
- Jue B., Maurice D. M. The mechanical properties of the rabbit and human cornea. J Biomech. 1986;19(10):847–853. doi: 10.1016/0021-9290(86)90135-1. [DOI] [PubMed] [Google Scholar]
- Kato T., Nakayasu K., Ikegami K., Obara T., Kanayama T., Kanai A. Analysis of glycosaminoglycans in rabbit cornea after excimer laser keratectomy. Br J Ophthalmol. 1999 May;83(5):609–612. doi: 10.1136/bjo.83.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Sah W. J., Kim M. S., Lee Y. C., Park C. K. Three-year results of photorefractive keratectomy for myopia. J Refract Surg. 1995 May-Jun;11(3 Suppl):S248–S252. doi: 10.3928/1081-597X-19950502-10. [DOI] [PubMed] [Google Scholar]
- Komai Y., Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991 Jul;32(8):2244–2258. [PubMed] [Google Scholar]
- Latvala T., Barraquer-Coll C., Tervo K., Tervo T. Corneal wound healing and nerve morphology after excimer laser in situ keratomileusis in human eyes. J Refract Surg. 1996 Sep-Oct;12(6):677–683. doi: 10.3928/1081-597X-19960901-08. [DOI] [PubMed] [Google Scholar]
- Latvala T., Barraquer-Coll C., Tervo K., Tervo T. Corneal wound healing and nerve morphology after excimer laser in situ keratomileusis in human eyes. J Refract Surg. 1996 Sep-Oct;12(6):677–683. doi: 10.3928/1081-597X-19960901-08. [DOI] [PubMed] [Google Scholar]
- Lin D. T. Corneal topographic analysis after excimer photorefractive keratectomy. Ophthalmology. 1994 Aug;101(8):1432–1439. doi: 10.1016/s0161-6420(94)31154-7. [DOI] [PubMed] [Google Scholar]
- Linna T. U., Vesaluoma M. H., Pérez-Santonja J. J., Petroll W. M., Alió J. L., Tervo T. M. Effect of myopic LASIK on corneal sensitivity and morphology of subbasal nerves. Invest Ophthalmol Vis Sci. 2000 Feb;41(2):393–397. [PubMed] [Google Scholar]
- Linna T., Tervo T. Real-time confocal microscopic observations on human corneal nerves and wound healing after excimer laser photorefractive keratectomy. Curr Eye Res. 1997 Jul;16(7):640–649. doi: 10.1076/ceyr.16.7.640.5058. [DOI] [PubMed] [Google Scholar]
- Maurice D. M., Monroe F. Cohesive strength of corneal lamellae. Exp Eye Res. 1990 Jan;50(1):59–63. doi: 10.1016/0014-4835(90)90011-i. [DOI] [PubMed] [Google Scholar]
- Møller-Pedersen T., Li H. F., Petroll W. M., Cavanagh H. D., Jester J. V. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998 Mar;39(3):487–501. [PubMed] [Google Scholar]
- Møller-Pedersen T., Vogel M., Li H. F., Petroll W. M., Cavanagh H. D., Jester J. V. Quantification of stromal thinning, epithelial thickness, and corneal haze after photorefractive keratectomy using in vivo confocal microscopy. Ophthalmology. 1997 Mar;104(3):360–368. doi: 10.1016/s0161-6420(97)30307-8. [DOI] [PubMed] [Google Scholar]
- Müller L. J., Pels L., Vrensen G. F. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995 Dec;36(13):2557–2567. [PubMed] [Google Scholar]
- Müller L. J., Pels L., Vrensen G. F. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996 Mar;37(4):476–488. [PubMed] [Google Scholar]
- Müller L. J., Vrensen G. F., Pels L., Cardozo B. N., Willekens B. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997 Apr;38(5):985–994. [PubMed] [Google Scholar]
- Patel S., Marshall J., Fitzke F. W., 3rd Refractive index of the human corneal epithelium and stroma. J Refract Surg. 1995 Mar-Apr;11(2):100–105. doi: 10.3928/1081-597X-19950301-09. [DOI] [PubMed] [Google Scholar]
- Pels E., Schuchard Y. The effects of high molecular weight dextran on the preservation of human corneas. Cornea. 1984;3(3):219–227. [PubMed] [Google Scholar]
- Pop M. Laser thermal keratoplasty for the treatment of photorefractive keratectomy overcorrections: a 1-year follow-up. Ophthalmology. 1998 May;105(5):926–931. doi: 10.1016/S0161-6420(98)95039-4. [DOI] [PubMed] [Google Scholar]
- Pouliquen Y. J. 1984 Castroviejo lecture. Fine structure of the corneal stroma. Cornea. 1984;3(3):168–177. [PubMed] [Google Scholar]
- Radner W., Zehetmayer M., Aufreiter R., Mallinger R. Interlacing and cross-angle distribution of collagen lamellae in the human cornea. Cornea. 1998 Sep;17(5):537–543. doi: 10.1097/00003226-199809000-00012. [DOI] [PubMed] [Google Scholar]
- Salah T., Waring G. O., 3rd, el Maghraby A., Moadel K., Grimm S. B. Excimer laser in situ keratomileusis under a corneal flap for myopia of 2 to 20 diopters. Am J Ophthalmol. 1996 Feb;121(2):143–155. [PubMed] [Google Scholar]
- Schallhorn S. C., Reid J. L., Kaupp S. E., Blanton C. L., Zoback L., Goforth H., Jr, Flowers C. W., McDonnell P. J. Topographic detection of photorefractive keratectomy. Ophthalmology. 1998 Mar;105(3):507–516. doi: 10.1016/S0161-6420(98)93035-4. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Keratan sulphate--a 'reserve' polysaccharide? Eur J Clin Chem Clin Biochem. 1994 Apr;32(4):217–223. [PubMed] [Google Scholar]
- Smolek M. K. Interlamellar cohesive strength in the vertical meridian of human eye bank corneas. Invest Ophthalmol Vis Sci. 1993 Sep;34(10):2962–2969. [PubMed] [Google Scholar]
- Stephenson C. G., Gartry D. S., O'Brart D. P., Kerr-Muir M. G., Marshall J. Photorefractive keratectomy. A 6-year follow-up study. Ophthalmology. 1998 Feb;105(2):273–281. doi: 10.1016/s0161-6420(98)93055-x. [DOI] [PubMed] [Google Scholar]
- Stulting R. D., Carr J. D., Thompson K. P., Waring G. O., 3rd, Wiley W. M., Walker J. G. Complications of laser in situ keratomileusis for the correction of myopia. Ophthalmology. 1999 Jan;106(1):13–20. doi: 10.1016/S0161-6420(99)90000-3. [DOI] [PubMed] [Google Scholar]
- Van Horn D. L., Doughman D. J., Harris J. E., Miller G. E., Lindstrom R., Good R. A. Ultrastructure of human organ-cultured cornea. II. Stroma and epithelium. Arch Ophthalmol. 1975 Apr;93(4):275–277. doi: 10.1001/archopht.1975.01010020285007. [DOI] [PubMed] [Google Scholar]
- Wigham C. G., Hodson S. A. Physiological changes in the cornea of the ageing eye. Eye (Lond) 1987;1(Pt 2):190–196. doi: 10.1038/eye.1987.36. [DOI] [PubMed] [Google Scholar]