Abstract
AIM—To evaluate the diagnostic value of the polymerase chain reaction (PCR) to detect Borrelia burgdorferi DNA in patients with ocular Lyme borreliosis. METHODS—Of 256 consecutive uveitis patients six selected individuals with clinical evidence for Lyme borreliosis and 30 patients with non-Lyme uveitis were enrolled. Lyme serology was performed by ELISA and western blotting. Urine samples were examined by an optimised nested polymerase chain reaction (PCR) protocol. RESULTS—Only four of six uveitis patients suspected for Lyme borreliosis were ELISA positive, while all six subjects showed a positive western blot. B burgdorferi PCR was positive in all of these six patients. Whereas two of the 30 controls had a positive Lyme serology, B burgdorferi DNA was not detectable by PCR in any sample from these patients. CONCLUSIONS—PCR for the detection of B burgdorferi DNA in urine of uveitis patients is a valuable tool to support the diagnosis of ocular Lyme borreliosis. Moreover, these patients often show a weak humoral immune response which may more sensitively be detected by immunoblotting.
Full Text
The Full Text of this article is available as a PDF (106.8 KB).
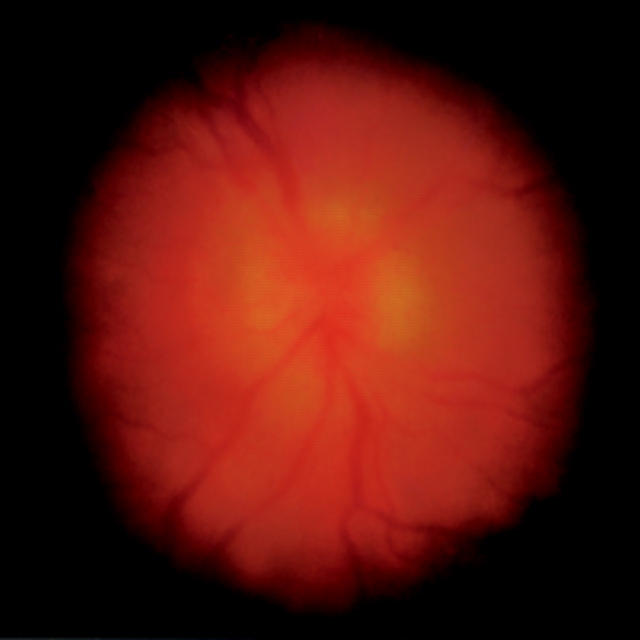
Figure 1 .
Fundus photograph. Patient 1 presenting with bilateral posterior uveitis, arthritis, and myalgia. Left eye at initial presentation (VA 20/40) with optic disc swelling, choroiditis, and vitritis. Seroblots were positive for immunoglobulin G and B burgdorferi DNA (p66) was detectable in urine of this patient.
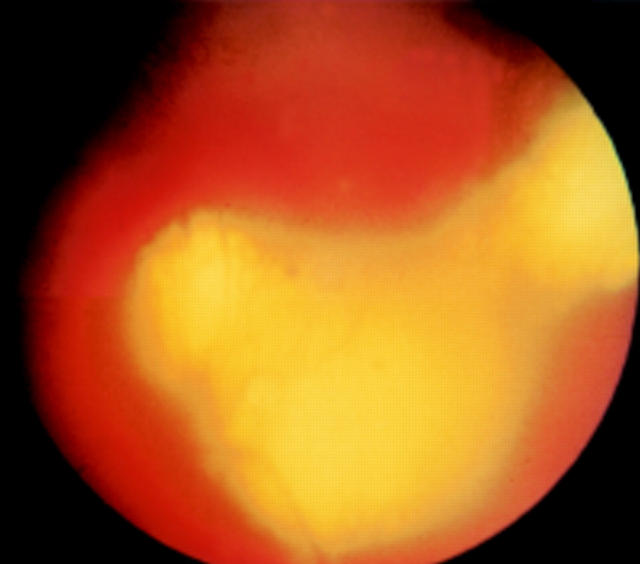
Figure 2 .
Patient 2 presenting with recurrent bilateral posterior uveitis and arthritis. Left eye at initial presentation (VA 20/80) with papillitis, optic disc swelling, and multiple choroidal lesions. A positive Lyme ELISA for IgG and western blots for IgM and IgG were detected. Urine PCR disclosed B burgdorferi DNA (ospA) that became negative following the initial treatment course. She developed recurrent uveitis, became PCR positive again during relapse of inflammation and eventually became stable after a second treatment course.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloch-Michel E., Nussenblatt R. B. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987 Feb 15;103(2):234–235. doi: 10.1016/s0002-9394(14)74235-7. [DOI] [PubMed] [Google Scholar]
- Dattwyler R. J., Volkman D. J., Luft B. J., Halperin J. J., Thomas J., Golightly M. G. Seronegative Lyme disease. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med. 1988 Dec 1;319(22):1441–1446. doi: 10.1056/NEJM198812013192203. [DOI] [PubMed] [Google Scholar]
- Gross D. M., Forsthuber T., Tary-Lehmann M., Etling C., Ito K., Nagy Z. A., Field J. A., Steere A. C., Huber B. T. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998 Jul 31;281(5377):703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- Hauser U., Lehnert G., Lobentanzer R., Wilske B. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J Clin Microbiol. 1997 Jun;35(6):1433–1444. doi: 10.1128/jcm.35.6.1433-1444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häupl T., Hahn G., Rittig M., Krause A., Schoerner C., Schönherr U., Kalden J. R., Burmester G. R. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 1993 Nov;36(11):1621–1626. doi: 10.1002/art.1780361118. [DOI] [PubMed] [Google Scholar]
- Jaulhac B., Nicolini P., Piemont Y., Monteil H. Detection of Borrelia burgdorferi in cerebrospinal fluid of patients with Lyme borreliosis. N Engl J Med. 1991 May 16;324(20):1440–1440. [PubMed] [Google Scholar]
- Karma A., Seppälä I., Mikkilä H., Kaakkola S., Viljanen M., Tarkkanen A. Diagnosis and clinical characteristics of ocular Lyme borreliosis. Am J Ophthalmol. 1995 Feb;119(2):127–135. doi: 10.1016/s0002-9394(14)73864-4. [DOI] [PubMed] [Google Scholar]
- Krause A., Brade V., Schoerner C., Solbach W., Kalden J. R., Burmester G. R. T cell proliferation induced by Borrelia burgdorferi in patients with Lyme borreliosis. Autologous serum required for optimum stimulation. Arthritis Rheum. 1991 Apr;34(4):393–402. doi: 10.1002/art.1780340404. [DOI] [PubMed] [Google Scholar]
- Krause A., Burmester G. R., Rensing A., Schoerner C., Schaible U. E., Simon M. M., Herzer P., Kramer M. D., Wallich R. Cellular immune reactivity to recombinant OspA and flagellin from Borrelia burgdorferi in patients with Lyme borreliosis. Complexity of humoral and cellular immune responses. J Clin Invest. 1992 Sep;90(3):1077–1084. doi: 10.1172/JCI115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölmel H. W., Neumann P., Schneider T., Barnick L., Lange R. Prevalence of antibodies to Borrelia burgdorferi in serum and cerebrospinal fluid samples from patients with neurological disorders in Berlin. Zentralbl Bakteriol. 1992 Dec;277(4):504–511. doi: 10.1016/s0934-8840(11)80475-5. [DOI] [PubMed] [Google Scholar]
- Lebech A. M., Hansen K. Detection of Borrelia burgdorferi DNA in urine samples and cerebrospinal fluid samples from patients with early and late Lyme neuroborreliosis by polymerase chain reaction. J Clin Microbiol. 1992 Jul;30(7):1646–1653. doi: 10.1128/jcm.30.7.1646-1653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Stafford K. C., 3rd Detection of Borrelia burgdorferi in urine of Peromyscus leucopus by inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1994 Mar;32(3):777–782. doi: 10.1128/jcm.32.3.777-782.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B. S., Chandler F. W., Felz M. W., Huey L. O., Field R. S. Detection of Borrelia burgdorferi in human blood and urine using the polymerase chain reaction. Pathobiology. 1992;60(3):163–167. doi: 10.1159/000163717. [DOI] [PubMed] [Google Scholar]
- Mouritsen C. L., Wittwer C. T., Litwin C. M., Yang L., Weis J. J., Martins T. B., Jaskowski T. D., Hill H. R. Polymerase chain reaction detection of Lyme disease: correlation with clinical manifestations and serologic responses. Am J Clin Pathol. 1996 May;105(5):647–654. doi: 10.1093/ajcp/105.5.647. [DOI] [PubMed] [Google Scholar]
- Preac-Mursic V., Pfister H. W., Spiegel H., Burk R., Wilske B., Reinhardt S., Böhmer R. First isolation of Borrelia burgdorferi from an iris biopsy. J Clin Neuroophthalmol. 1993 Sep;13(3):155–162. [PubMed] [Google Scholar]
- Priem S., Rittig M. G., Kamradt T., Burmester G. R., Krause A. An optimized PCR leads to rapid and highly sensitive detection of Borrelia burgdorferi in patients with Lyme borreliosis. J Clin Microbiol. 1997 Mar;35(3):685–690. doi: 10.1128/jcm.35.3.685-690.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn D. W., Malawista S. E. Lyme disease: recommendations for diagnosis and treatment. Ann Intern Med. 1991 Mar 15;114(6):472–481. doi: 10.7326/0003-4819-114-6-472. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. T., Rahn D. W. Prevalence of Lyme disease among patients with uveitis. Am J Ophthalmol. 1991 Oct 15;112(4):462–463. doi: 10.1016/s0002-9394(14)76262-2. [DOI] [PubMed] [Google Scholar]
- Schmidt B. L. PCR in laboratory diagnosis of human Borrelia burgdorferi infections. Clin Microbiol Rev. 1997 Jan;10(1):185–201. doi: 10.1128/cmr.10.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Schrumpf M. E., Karstens R. H. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J Clin Microbiol. 1988 May;26(5):893–895. doi: 10.1128/jcm.26.5.893-895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam A. P., Kuiper H., Vos K., Widjojokusumo A., de Jongh B. M., Spanjaard L., Ramselaar A. C., Kramer M. D., Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993 Oct;17(4):708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]