Abstract
BACKGROUND/AIMS—The Arthus type allergic reaction is characterised by inflammatory cell infiltration and marked neovascularisation in the cornea. During the healing stages, inflammatory cells and newly formed microvessels gradually disappear. The aim was to establish whether apoptosis affected the regression of inflammatory cells and newly formed microvessels, in order to define more clearly the cellular mechanisms involved in the pathobiology of corneal diseases. METHODS—Albino male rabbits were injected subcutaneously with 5 mg/ml bovine serum albumin (BSA) incorporated in Freund's complete adjuvant twice weekly. Under the anaesthesia, 30 µl of a 0.5 mg/ml BSA solution was injected into the central corneal stroma to induce an Arthus type allergic reaction. The injured corneas were collected at various time points ranging from 3 to 20 days. Apoptotic cells were identified by both light microscopy using in situ TdT-dUTP nick end labelling (TUNEL) method and electron microscopy. RESULTS—With increasing time after induction of the Arthus reaction, marked neovascularisation and infiltrated inflammatory cells such as polymorphonuclear cells (PMNs) and plasma cells were observed in the cornea. Thereafter, the inflammatory cells and newly formed microvessels gradually disappeared. Coincidently, the numbers of microvessel endothelial cells and infiltrated inflammatory cells undergoing apoptosis were increased. Apoptotic bodies were taken up by macrophages, PMNs, as well as myofibroblasts derived presumably from transformation of migrated keratocytes. CONCLUSIONS—These data demonstrate that regression of the cellular infiltrates and microvessel endothelial cells associated with the Arthus reaction in the cornea occurs via apoptosis. This finding adds insights into the cellular mechanisms regulating the pathobiology of corneal diseases.
Full Text
The Full Text of this article is available as a PDF (315.2 KB).
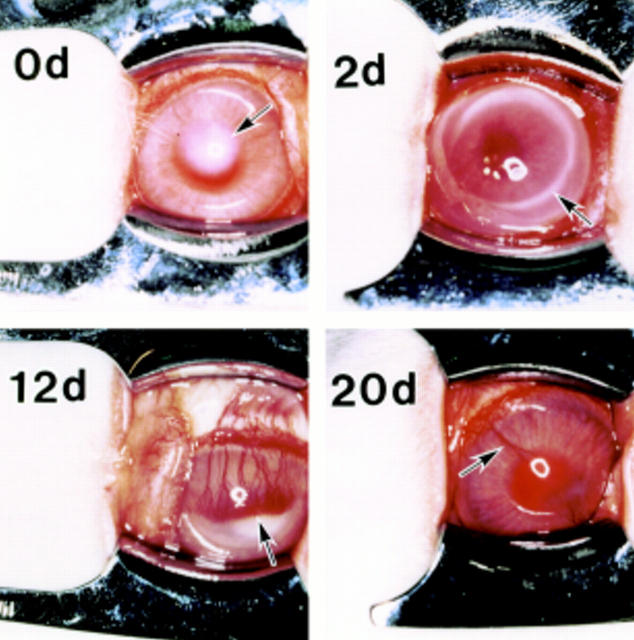
Figure 1 .
Sensitised rabbit corneas showing opacity as a result of injection (arrow) at day 0 (0d), immuno-ring (arrow) at 2 days (2d) after injection and induction of neovascular response. Newly formed microvessels (arrow) reached to the centre of the cornea at 12 days (12d). These microvessels (arrow) gradually diminished at the healing stage on day 20 (20d).
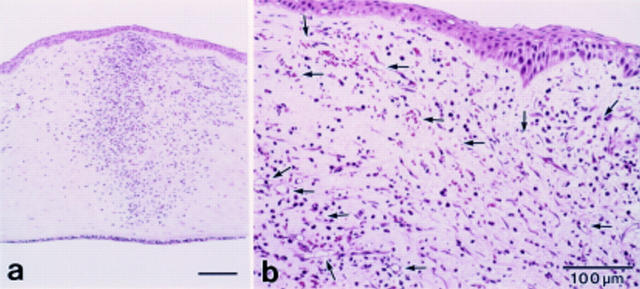
Figure 2 .
Light microscopic observation (haematoxylin eosin staining) at 3 days. (a) The infiltration of inflammatory cells mainly polymorphonuclear neutrophils (PMNs) was noted. (b) At the limbus newly formed dense capillaries were observed (arrows).
Figure 3 .
Double staining for apoptotic cells, and microvessels in the same section at 3 days after injection of BSA. TUNEL positive cells (green, arrowheads) were present in the stroma. However, there were no positive cells in the microvessels as depicted by positive reaction for TM (red, arrows).
Figure 4 .
Double staining for apoptotic cells, and microvessels in the same section at 12 days after injection of BSA. Co-localisation of TM expression (red, arrows) and TUNEL positivity (green, arrowheads)is seen in the endothelium.
Figure 5 .

Various types of apoptotic cells and bodies in the stroma are shown (arrows).
Figure 6 .

A plasma cell in the early stage of apoptosis at 9 days after injection of BSA.
Figure 7 .

Apoptotic cell in microvessel at 12 days after injection of BSA showing an endothelial cell in the early stage of apoptosis. Note condensation of heterochromatin to form "crescent" in the lobes of the nucleus (*). Inset shows pinocytic vesicle of endothelial cells (arrows).
Figure 8 .

Phagocytosis of apoptotic cells at 12 days after injection of BSA. (A) A macrophage containing apparently a PMN with granules and fragments of chromatin condensation typical of apoptosis (arrows). (B) A PMN containing apparently a neutrophil with granules and fragments of chromatin condensation typical of apoptosis (arrows). (C) Apoptotic body (large arrows) was taken up by fibroblastic cells. This fibroblastic cell has extracellular microtendon associated with the fibronexis (small arrows), electron dense stress fibre (arrowheads), and abundant rough ER, suggesting a myofibroblast.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coles H. S., Burne J. F., Raff M. C. Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development. 1993 Jul;118(3):777–784. doi: 10.1242/dev.118.3.777. [DOI] [PubMed] [Google Scholar]
- DeBault L. E., Esmon N. L., Olson J. R., Esmon C. T. Distribution of the thrombomodulin antigen in the rabbit vasculature. Lab Invest. 1986 Feb;54(2):172–178. [PubMed] [Google Scholar]
- Desmoulière A., Badid C., Bochaton-Piallat M. L., Gabbiani G. Apoptosis during wound healing, fibrocontractive diseases and vascular wall injury. Int J Biochem Cell Biol. 1997 Jan;29(1):19–30. doi: 10.1016/s1357-2725(96)00117-3. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobé G., Browning J., Howard T., Hogg N., Winterford C., Cross R. Apoptosis occurs in endothelial cells during hypertension-induced microvascular rarefaction. J Struct Biol. 1997 Feb;118(1):63–72. doi: 10.1006/jsbi.1996.3835. [DOI] [PubMed] [Google Scholar]
- Griffith T. S., Brunner T., Fletcher S. M., Green D. R., Ferguson T. A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995 Nov 17;270(5239):1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- Horvat R., Palade G. E. Thrombomodulin and thrombin localization on the vascular endothelium; their internalization and transcytosis by plasmalemmal vesicles. Eur J Cell Biol. 1993 Aug;61(2):299–313. [PubMed] [Google Scholar]
- Ishizaki M., Zhu G., Haseba T., Shafer S. S., Kao W. W. Expression of collagen I, smooth muscle alpha-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest Ophthalmol Vis Sci. 1993 Nov;34(12):3320–3328. [PubMed] [Google Scholar]
- Jones J., Morgan B. P. Apoptosis is associated with reduced expression of complement regulatory molecules, adhesion molecules and other receptors on polymorphonuclear leucocytes: functional relevance and role in inflammation. Immunology. 1995 Dec;86(4):651–660. [PMC free article] [PubMed] [Google Scholar]
- Kilgore K. S., Flory C. M., Miller B. F., Evans V. M., Warren J. S. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol. 1996 Sep;149(3):953–961. [PMC free article] [PubMed] [Google Scholar]
- Liles W. C., Kiener P. A., Ledbetter J. A., Aruffo A., Klebanoff S. J. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996 Aug 1;184(2):429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Marshall J. C., Watson R. W. Programmed cell death (apoptosis) and the resolution of systemic inflammation. Can J Surg. 1997 Jun;40(3):169–174. [PMC free article] [PubMed] [Google Scholar]
- Meeson A., Palmer M., Calfon M., Lang R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996 Dec;122(12):3929–3938. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997 Feb 7;88(3):355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Nishiuchi R., Yoshino T., Matsuo Y., Sakuma I., Cao L., Seino Y., Takahashi K., Akagi T. The Fas antigen is detected on immature B cells and the representative cell lines show Fas-mediated apoptosis. Br J Haematol. 1996 Feb;92(2):302–307. doi: 10.1046/j.1365-2141.1996.d01-1463.x. [DOI] [PubMed] [Google Scholar]
- Pollman M. J., Naumovski L., Gibbons G. H. Endothelial cell apoptosis in capillary network remodeling. J Cell Physiol. 1999 Mar;178(3):359–370. doi: 10.1002/(SICI)1097-4652(199903)178:3<359::AID-JCP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sang Q. X. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998 Sep;8(3):171–177. doi: 10.1038/cr.1998.17. [DOI] [PubMed] [Google Scholar]
- Streilein J. W. Unraveling immune privilege. Science. 1995 Nov 17;270(5239):1158–1159. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Griffith T. S., Usui N., Pepose J., Yu X., Ferguson T. A. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997 Feb 1;99(3):396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Kaji K., Nagasawa S. Activation of the alternative pathway of human complement by apoptotic human umbilical vein endothelial cells. J Biochem. 1994 Oct;116(4):794–800. doi: 10.1093/oxfordjournals.jbchem.a124598. [DOI] [PubMed] [Google Scholar]
- Wilson S. E., Kim W. J. Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest Ophthalmol Vis Sci. 1998 Feb;39(2):220–226. [PubMed] [Google Scholar]