Abstract
AIMS—To compare the efficacy and safety of famciclovir with aciclovir for the treatment of ophthalmic zoster. METHODS—Randomised, double masked, aciclovir controlled, parallel group in 87 centres worldwide including 454 patients with ophthalmic zoster of trigeminal nerve (V1) comprised the intent to treat population. Oral famciclovir 500 mg three times daily or oral aciclovir 800 mg five times daily for 7 days. Assessments included day 0 (screening), days 3 and 7 (during treatment), days 10, 14, 21, 28 and monthly thereafter, up to 6 months (follow up). Proportion of patients who experienced ocular manifestations, severe manifestations and non-severe manifestations; loss of visual acuity was the main outcome measure. RESULTS—The percentage of patients who experienced one or more ocular manifestations was similar for famciclovir (142/245, 58.0%) and aciclovir (114/196, 58.2%) recipients, with no significant difference between groups (OR 0.99; 95% CI 0.68, 1.45). The percentage of patients who experienced severe and non-severe manifestations was similar between groups, with no significant difference. The prevalence of individual ocular manifestations was comparable between groups. There was no significant difference between groups for visual acuity loss. CONCLUSION—Famciclovir 500 mg three times daily was well tolerated and demonstrated efficacy similar to aciclovir 800 mg five times daily.
Full Text
The Full Text of this article is available as a PDF (137.9 KB).
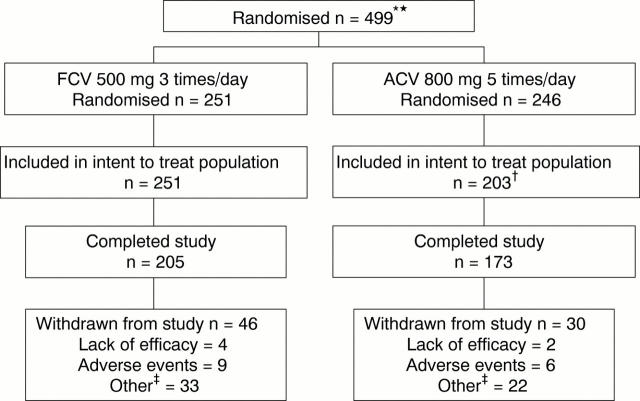
Figure 1 .
Trial profile. *Includes two patients who were randomised but never received medication. †43 patients received non-bioequivalent aciclovir and thus were excluded from the intent to treat population. ‡"Other" reasons included lack of patient compliance, patient lost to follow up, and protocol violation.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cobo L. M., Foulks G. N., Liesegang T., Lass J., Sutphin J. E., Wilhelmus K., Jones D. B., Chapman S., Segreti A. C., King D. H. Oral acyclovir in the treatment of acute herpes zoster ophthalmicus. Ophthalmology. 1986 Jun;93(6):763–770. doi: 10.1016/s0161-6420(86)33678-9. [DOI] [PubMed] [Google Scholar]
- Harding S. P., Lipton J. R., Wells J. C. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987 May;71(5):353–358. doi: 10.1136/bjo.71.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. P. Management of ophthalmic zoster. J Med Virol. 1993;Suppl 1:97–101. doi: 10.1002/jmv.1890410519. [DOI] [PubMed] [Google Scholar]
- Harding S. P., Porter S. M. Oral acyclovir in herpes zoster ophthalmicus. Curr Eye Res. 1991;10 (Suppl):177–182. doi: 10.3109/02713689109020376. [DOI] [PubMed] [Google Scholar]
- Hoang-Xuan T., Büchi E. R., Herbort C. P., Denis J., Frot P., Thénault S., Pouliquen Y. Oral acyclovir for herpes zoster ophthalmicus. Ophthalmology. 1992 Jul;99(7):1062–1071. doi: 10.1016/s0161-6420(92)31849-4. [DOI] [PubMed] [Google Scholar]
- McKendrick M. W., McGill J. I., White J. E., Wood M. J. Oral acyclovir in acute herpes zoster. Br Med J (Clin Res Ed) 1986 Dec 13;293(6561):1529–1532. doi: 10.1136/bmj.293.6561.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton P., Thomson A. N. Oral acyclovir in the treatment of herpes zoster in general practice. N Z Med J. 1989 Mar 8;102(863):93–95. [PubMed] [Google Scholar]
- O'Brien J. J., Campoli-Richards D. M. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1989 Mar;37(3):233–309. doi: 10.2165/00003495-198937030-00002. [DOI] [PubMed] [Google Scholar]
- Pue M. A., Pratt S. K., Fairless A. J., Fowles S., Laroche J., Georgiou P., Prince W. Linear pharmacokinetics of penciclovir following administration of single oral doses of famciclovir 125, 250, 500 and 750 mg to healthy volunteers. J Antimicrob Chemother. 1994 Jan;33(1):119–127. doi: 10.1093/jac/33.1.119. [DOI] [PubMed] [Google Scholar]
- Tyring S., Barbarash R. A., Nahlik J. E., Cunningham A., Marley J., Heng M., Jones T., Rea T., Boon R., Saltzman R. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995 Jul 15;123(2):89–96. doi: 10.7326/0003-4819-123-2-199507150-00002. [DOI] [PubMed] [Google Scholar]
- Wade J. C., Newton B., Flournoy N., Meyers J. D. Oral acyclovir for prevention of herpes simplex virus reactivation after marrow transplantation. Ann Intern Med. 1984 Jun;100(6):823–828. doi: 10.7326/0003-4819-100-6-823. [DOI] [PubMed] [Google Scholar]
- Womack L. W., Liesegang T. J. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983 Jan;101(1):42–45. doi: 10.1001/archopht.1983.01040010044004. [DOI] [PubMed] [Google Scholar]
- de Miranda P., Blum M. R. Pharmacokinetics of acyclovir after intravenous and oral administration. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):29–37. doi: 10.1093/jac/12.suppl_b.29. [DOI] [PubMed] [Google Scholar]