Abstract
BACKGROUND/AIMS—Posterior uveal melanoma is the most common intraocular tumour in adults, responsible for the death of approximately 35% of patients. Hepatic metastases are most frequent, and once diagnosed survival is usually less than 1 year. The β1 family of integrins, αvβ3 and MMP-2 and MMP-9 have been implicated in the metastasis of several types of tumour. To study their involvement in uveal melanoma we analysed the expression of the β1 integrins, αvβ3, MMP-2, and MMP-9 in 10 primary posterior uveal melanomas, and correlated expression with invasive potential in vitro. Comparable studies were undertaken on cultures of melanocytes. METHODS—Expression of integrins was studied by immunohistochemistry, secretion of MMP-2 and MMP-9 by zymography, and the invasive potential was assessed using a transwell model. RESULTS—MMP-2 was secreted by all uveal melanomas and seven of 10 secreted MMP-9. Among uveal melanoma, invasion levels of 4-25% were observed and the major integrins expressed were α1β1, α2β1, α3β1, α5β1, and avβ3. Melanocytes did not express α1β1, α4β1, and α6β1. CONCLUSION—The laminin binding α6β1 integrin was not expressed by either melanocytes or tumours with spindle morphology, which are considered to have a better prognosis. It is possible that expression of the α6β1 integrin may prove useful as a prognostic indicator.
Full Text
The Full Text of this article is available as a PDF (227.0 KB).
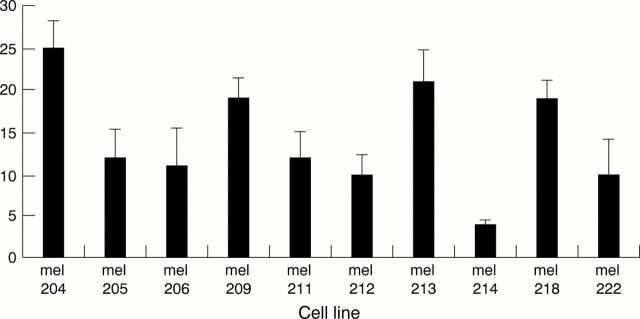
Figure 1 .
Percentage invasion of 10 cultures of primary posterior uveal melanoma as determined by invasion assay. All cultures were tested within the first five passages. Values shown are means (SEM) for three combined assays.
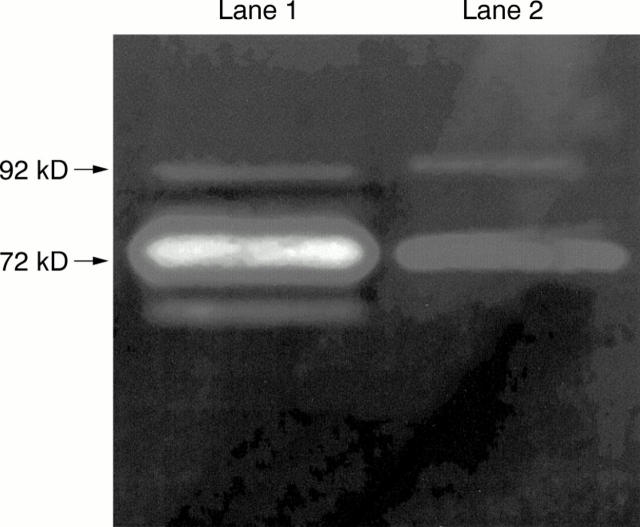
Figure 2 .
Gelatin zymogram showing examples of MMP-2 (72 kD) and MMP-9 (92 kD) secretion by cultured posterior uveal melanoma cells. A molecular weight marker was run on each gel (not shown). Mel 218 (lane 1) also secreted the active forms of both enzymes. Mel 222 (lane 2).
Figure 3 .
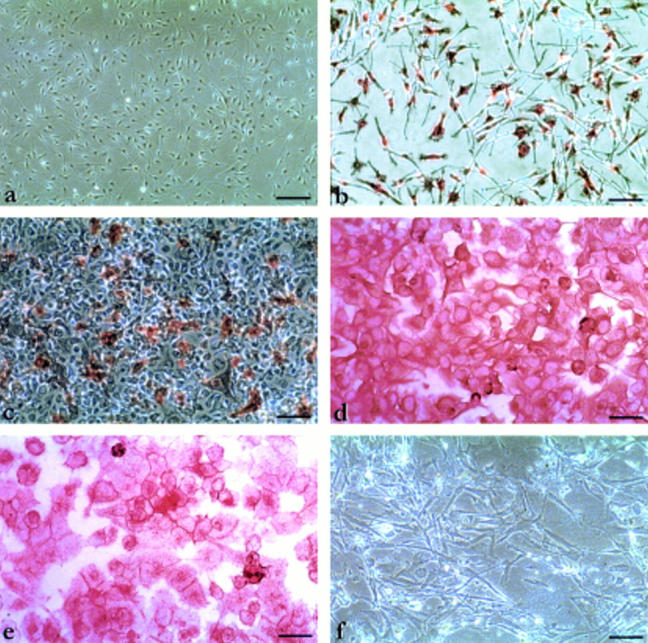
Photomicrographs showing examples of immunohistochemical staining for integrin expression in cultures of posterior uveal melanoma and melanocytes. (a) Negative staining for α1β1 in melanocytes (×10) (scale bar 100 µm). (b) Homogeneous staining for α3β1 in melanocytes (×40) (scale bar 400 µm). (c) Positive heterogeneous staining of x% of cells for α1β1 in melanoma cells with epithelioid morphology (×20) (scale bar 200 µm). (d) Homogeneous staining for αvβ3 in melanoma cells with epithelioid morphology (×40) (scale bar 400 µm). (e) Homogeneous staining for α6β1 in melanoma cells with epithelioid morphology (×40) (scale bar 400 µm). (f) Negative staining for α6β1 in melanoma cells with spindle-like morphology (×40) (scale bar 400 µm).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrez M., Gu X., Turton J., Meldrum C., Niu J., Antalis T., Howard E. W. The alpha v beta 6 integrin induces gelatinase B secretion in colon cancer cells. Int J Cancer. 1999 Mar 31;81(1):90–97. doi: 10.1002/(sici)1097-0215(19990331)81:1<90::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Strömblad S., Sanders L. C., von Schalscha T. L., Aimes R. T., Stetler-Stevenson W. G., Quigley J. P., Cheresh D. A. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996 May 31;85(5):683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Campo E., Merino M. J., Tavassoli F. A., Charonis A. S., Stetler-Stevenson W. G., Liotta L. A. Evaluation of basement membrane components and the 72 kDa type IV collagenase in serous tumors of the ovary. Am J Surg Pathol. 1992 May;16(5):500–507. doi: 10.1097/00000478-199205000-00009. [DOI] [PubMed] [Google Scholar]
- Char D. H. Metastatic choroidal melanoma. Am J Ophthalmol. 1978 Jul;86(1):76–80. doi: 10.1016/0002-9394(78)90018-1. [DOI] [PubMed] [Google Scholar]
- Clavel C., Polette M., Doco M., Binninger I., Birembaut P. Immunolocalization of matrix metallo-proteinases and their tissue inhibitor in human mammary pathology. Bull Cancer. 1992;79(3):261–270. [PubMed] [Google Scholar]
- Cottam D. W., Rennie I. G., Woods K., Parsons M. A., Bunning R. A., Rees R. C. Gelatinolytic metalloproteinase secretion patterns in ocular melanoma. Invest Ophthalmol Vis Sci. 1992 May;33(6):1923–1927. [PubMed] [Google Scholar]
- Creyghton W. M., de Waard-Siebinga I., Danen E. H., Luyten G. P., van Muijen G. N., Jager M. J. Cytokine-mediated modulation of integrin, ICAM-1 and CD44 expression on human uveal melanoma cells in vitro. Melanoma Res. 1995 Aug;5(4):235–242. doi: 10.1097/00008390-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Danen E. H., Ten Berge P. J., Van Muijen G. N., Van 't Hof-Grootenboer A. E., Bröcker E. B., Ruiter D. J. Emergence of alpha 5 beta 1 fibronectin- and alpha v beta 3 vitronectin-receptor expression in melanocytic tumour progression. Histopathology. 1994 Mar;24(3):249–256. doi: 10.1111/j.1365-2559.1994.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Dewhurst L. O., Gee J. W., Rennie I. G., MacNeil S. Tamoxifen, 17beta-oestradiol and the calmodulin antagonist J8 inhibit human melanoma cell invasion through fibronectin. Br J Cancer. 1997;75(6):860–868. doi: 10.1038/bjc.1997.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan K. M., Seddon J. M., Glynn R. J., Gragoudas E. S., Albert D. M. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988 Jan-Feb;32(4):239–251. doi: 10.1016/0039-6257(88)90173-7. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Falcioni R., Kennel S. J., Giacomini P., Zupi G., Sacchi A. Expression of tumor antigen correlated with metastatic potential of Lewis lung carcinoma and B16 melanoma clones in mice. Cancer Res. 1986 Nov;46(11):5772–5778. [PubMed] [Google Scholar]
- Gehlsen K. R., Davis G. E., Sriramarao P. Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin Exp Metastasis. 1992 Mar;10(2):111–120. doi: 10.1007/BF00114587. [DOI] [PubMed] [Google Scholar]
- Gouon V., Tucker G. C., Kraus-Berthier L., Atassi G., Kieffer N. Up-regulated expression of the beta3 integrin and the 92-kDa gelatinase in human HT-144 melanoma cell tumors grown in nude mice. Int J Cancer. 1996 Nov 27;68(5):650–662. doi: 10.1002/(SICI)1097-0215(19961127)68:5<650::AID-IJC16>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Gragoudas E. S., Egan K. M., Seddon J. M., Glynn R. J., Walsh S. M., Finn S. M., Munzenrider J. E., Spar M. D. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991 Mar;98(3):383–390. doi: 10.1016/s0161-6420(91)32285-1. [DOI] [PubMed] [Google Scholar]
- Hart I. R., Saini A. Biology of tumour metastasis. Lancet. 1992 Jun 13;339(8807):1453–1457. doi: 10.1016/0140-6736(92)92039-i. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981 Jun;75(6):816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y., Robey F. A., Graf J., Sasaki M., Kleinman H. K., Yamada Y., Martin G. R. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987 Nov 20;238(4830):1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Khokha R., Zimmer M. J., Graham C. H., Lala P. K., Waterhouse P. Suppression of invasion by inducible expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in B16-F10 melanoma cells. J Natl Cancer Inst. 1992 Jul 1;84(13):1017–1022. doi: 10.1093/jnci/84.13.1017. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Vu M. P., Cheng Y. F., Ramos D. M., Timpl R., Waleh N. Laminin-binding integrin alpha 7 beta 1: functional characterization and expression in normal and malignant melanocytes. Cell Regul. 1991 Oct;2(10):805–817. doi: 10.1091/mbc.2.10.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy A. T., Cioce V., Sobel M. E., Garbisa S., Grigioni W. F., Liotta L. A., Stetler-Stevenson W. G. Increased expression of the Mr 72,000 type IV collagenase in human colonic adenocarcinoma. Cancer Res. 1991 Jan 1;51(1):439–444. [PubMed] [Google Scholar]
- Lotz M. M., Korzelius C. A., Mercurio A. M. Human colon carcinoma cells use multiple receptors to adhere to laminin: involvement of alpha 6 beta 4 and alpha 2 beta 1 integrins. Cell Regul. 1990 Feb;1(3):249–257. doi: 10.1091/mbc.1.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. F., Rutherford D. C., Happerfield L., Hanby A., McCartney A. C., Newton-Bishop J., Hart I. R. Comparative analysis of integrins in vitro and in vivo in uveal and cutaneous melanomas. Br J Cancer. 1998 Feb;77(4):522–529. doi: 10.1038/bjc.1998.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M. J., Foster W. D., Zimmerman L. E. Prognostic factors in small malignant melanomas of choroid and ciliary body. Arch Ophthalmol. 1977 Jan;95(1):48–58. doi: 10.1001/archopht.1977.04450010050004. [DOI] [PubMed] [Google Scholar]
- Moretti S., Martini L., Berti E., Pinzi C., Giannotti B. Adhesion molecule profile and malignancy of melanocytic lesions. Melanoma Res. 1993 Aug;3(4):235–239. [PubMed] [Google Scholar]
- Mortarini R., Anichini A. From adhesion to signalling: roles of integrins in the biology of human melanoma. Melanoma Res. 1993 Apr;3(2):87–97. [PubMed] [Google Scholar]
- Natali P. G., Hamby C. V., Felding-Habermann B., Liang B., Nicotra M. R., Di Filippo F., Giannarelli D., Temponi M., Ferrone S. Clinical significance of alpha(v)beta3 integrin and intercellular adhesion molecule-1 expression in cutaneous malignant melanoma lesions. Cancer Res. 1997 Apr 15;57(8):1554–1560. [PubMed] [Google Scholar]
- Pignatelli M., Vessey C. J. Adhesion molecules: novel molecular tools in tumor pathology. Hum Pathol. 1994 Sep;25(9):849–856. doi: 10.1016/0046-8177(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Riikonen T., Westermarck J., Koivisto L., Broberg A., Kähäri V. M., Heino J. Integrin alpha 2 beta 1 is a positive regulator of collagenase (MMP-1) and collagen alpha 1(I) gene expression. J Biol Chem. 1995 Jun 2;270(22):13548–13552. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- Rohrbach J. M., Wild M., Riedinger C., Kreissig I., Thiel H. J. Premetastatic uveal melanoma cells do not express laminin receptors. Ger J Ophthalmol. 1994 May;3(3):144–147. [PubMed] [Google Scholar]
- Seddon J. M., Albert D. M., Lavin P. T., Robinson N. A prognostic factor study of disease-free interval and survival following enucleation for uveal melanoma. Arch Ophthalmol. 1983 Dec;101(12):1894–1899. doi: 10.1001/archopht.1983.01040020896012. [DOI] [PubMed] [Google Scholar]
- Seftor R. E., Seftor E. A., Gehlsen K. R., Stetler-Stevenson W. G., Brown P. D., Ruoslahti E., Hendrix M. J. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammas H. F., Blodi F. C. Prognostic factors in choroidal and ciliary body melanomas. Arch Ophthalmol. 1977 Jan;95(1):63–69. doi: 10.1001/archopht.1977.04450010065005. [DOI] [PubMed] [Google Scholar]
- Shields J. A., Shields C. L., Donoso L. A. Management of posterior uveal melanoma. Surv Ophthalmol. 1991 Nov-Dec;36(3):161–195. doi: 10.1016/0039-6257(91)90001-v. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Thomas L., Chan P. W., Chang S., Damsky C. 5-Bromo-2-deoxyuridine regulates invasiveness and expression of integrins and matrix-degrading proteinases in a differentiated hamster melanoma cell. J Cell Sci. 1993 May;105(Pt 1):191–201. doi: 10.1242/jcs.105.1.191. [DOI] [PubMed] [Google Scholar]
- Urbanski S. J., Edwards D. R., Maitland A., Leco K. J., Watson A., Kossakowska A. E. Expression of metalloproteinases and their inhibitors in primary pulmonary carcinomas. Br J Cancer. 1992 Dec;66(6):1188–1194. doi: 10.1038/bjc.1992.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann R., Kreuser E. D., Adler G., Lutz M. P. Integrin alpha6beta1 role in metastatic behavior of human pancreatic carcinoma cells. Int J Cancer. 1999 Mar 1;80(5):791–795. doi: 10.1002/(sici)1097-0215(19990301)80:5<791::aid-ijc25>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Väisänen A., Kallioinen M., von Dickhoff K., Laatikainen L., Höyhtyä M., Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (MMP-2) immunoreactive protein--a new prognostic marker in uveal melanoma? J Pathol. 1999 May;188(1):56–62. doi: 10.1002/(SICI)1096-9896(199905)188:1<56::AID-PATH304>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Wagner M., Bielby S., Rennie I. G., Mac Neil S. Attachment of human uveal melanocytes and melanoma cells to extracellular matrix proteins involves intracellular calcium and calmodulin. Melanoma Res. 1997 Dec;7(6):439–448. doi: 10.1097/00008390-199712000-00001. [DOI] [PubMed] [Google Scholar]
- Wang M. X., Shields J. A., Donoso L. A. Subclinical metastasis of uveal melanoma. Int Ophthalmol Clin. 1993 Summer;33(3):119–127. doi: 10.1097/00004397-199303330-00017. [DOI] [PubMed] [Google Scholar]
- Zetter B. R. Adhesion molecules in tumor metastasis. Semin Cancer Biol. 1993 Aug;4(4):219–229. [PubMed] [Google Scholar]
- Zimmerman L. E. Metastatic disease from uveal melanomas. A review of current concepts with comments concerning future research and prevention. Trans Ophthalmol Soc U K. 1980 Apr;100(Pt 1):34–54. [PubMed] [Google Scholar]
- ten Berge P. J., Danen E. H., van Muijen G. N., Jager M. J., Ruiter D. J. Integrin expression in uveal melanoma differs from cutaneous melanoma. Invest Ophthalmol Vis Sci. 1993 Dec;34(13):3635–3640. [PubMed] [Google Scholar]