Abstract
The neuropeptide galanin is expressed developmentally in the dorsal root ganglion (DRG) and is rapidly up-regulated 120-fold after peripheral nerve section in the adult. Here we report that adult mice carrying a loss-of-function mutation in the galanin gene have a 13% reduction in the number of cells in the DRG associated with a 24% decrease in the percentage of neurons that express substance P. These deficits are associated with a 2.8- and 2.6-fold increase in the number of apoptotic cells in the DRG at postnatal days 3 and 4, respectively. After crush injury to the sciatic nerve, the rate of peripheral nerve regeneration is reduced by 35% with associated long-term functional deficits. Cultured DRG neurons from adult mutant mice demonstrate similar deficits in neurite number and length. These results identify a critical role for galanin in the development and regeneration of sensory neurons.
Damage to a peripheral nerve causes changes within the cell body that promote neuronal survival, axonal regeneration, and functional recovery. Under favorable conditions, for instance after a crush injury, most nerve fibers successfully regenerate. However, in many clinically relevant circumstances, traumatic or disease-induced nerve injury has a poor outcome with only limited return of function and often with considerable delay. The molecular and cellular interactions that control the degree and rate of peripheral nerve regeneration are poorly understood and remain important clinical and scientific issues.
In an attempt to define the mechanisms that regulate neuronal survival and regeneration, we and others have used the approach of studying factors whose expression patterns are known to change in response to injury. One of the most potent changes in the dorsal root ganglion (DRG) after peripheral nerve injury is the 120-fold increase in the levels of the 29-aa neuropeptide galanin (1). Studies have demonstrated that galanin is expressed at high levels in most cells of the developing DRG from day 16 of gestation until shortly after birth (2). In the adult, galanin is expressed at low levels in only 2–3% of DRG cells, which are predominantly small fiber neurons (1). After axotomy, mRNA and peptide are abundantly expressed in 40–50% of all DRG neurons (3) and remain elevated while the nerve is regenerating (1). Similarly, axotomy also up-regulates galanin expression in motor (4) and sympathetic (5) neurons. The rise in expression in the dorsal horn after axotomy is modest compared with the marked elevation in the DRG (6), reflecting an increase in anterograde transport of galanin from the cell body to the site of injury (7), analogous to that described in axotomized sympathetic neurons (8). Despite these findings, there is no direct evidence that galanin plays a role in axonal regeneration after injury.
We previously have generated mice carrying a loss-of-function mutation in the galanin gene (9) and most recently have demonstrated that the chronic absence of galanin throughout prenatal and postnatal development causes an attenuation in chronic neuropathic pain behavior (10). To investigate the role of galanin in the somatosensory system after injury we examined the cell bodies and axons of the sensory neurons themselves. In this paper we describe developmental deficits in the small peptidergic DRG neurons of the galanin mutant animals. There is a wave of cell loss that occurs in the DRG at postnatal days 3 and 4 (P3/4) when the number of apoptotic cells in the mutant DRG is approximately 2.5-fold higher than that observed in the wild-type controls. The rate of regeneration in the adult after a crush injury to the sciatic nerve is reduced in these animals, leading to long-term sensorimotor deficits. These in vivo findings are paralleled by a significant reduction in the number and the length of neurites from mutant DRG cells in culture.
Methods
Animals.
All experiments were performed on mice homozygous for a targeted mutation in the galanin gene. Age- and sex-matched wild-type littermates were used as controls in all experiments. Details of the exact strain and breeding history of the colony have been published (9, 10). In brief, the generation of the galanin knockout mice was performed by using the E14 cell line, and the colony has remained in-bred on the 129olaHsd strain and is currently at F16. All animals were fed standard chow and water ad libitum. Animal care and procedures were carried out within United Kingdom home-office protocols and guidelines. Animals were anaesthetized with sodium pentobarbitone (Sagatal, 60 mg/kg i.p.). In all cases, experiments and data analysis were performed with the observer blinded to the genotype of the animals studied. Data are presented as mean ± SEM.
Stereological and Histological Analysis.
For stereological cell counting DRG was embedded in paraffin, and 4-μm sections were stained with toluidine blue. The numbers of neurons in each DRG was determined by using the physical dissector method. Specifically, in each DRG, pairs of adjacent sections (reference section and look-up section) were compared. Nuclei that appeared in the reference section but not the look-up section (so-called cell tops) were counted. For each DRG, this procedure was done for every eighth pair of sections. To determine the first dissector pair to be used for each DRG a number from 1 to 8 was selected randomly. The number of cells in all sections pairs counted was divided by the summed volume of the two sections making up each section pair to determine the density of cells. Total numbers of cells in the L4 and L5 DRG then were determined by multiplying this density measure by the total DRG volume (11).
For immunocytochemistry, animals were anaesthetized and killed by perfusion with 4% paraformaldehyde, tissue was equilibrated overnight in 15% sucrose, and 6-μm frozen sections was cut on a cryostat. Sections were stained by using primary antisera against calcitonin gene-related peptide (CGRP), substance P, and neurofilament by standard diaminobenzidine (DAB) methods (i.e., primary antibody overnight; 2 × 15-min washes, biotinylated secondary antibody at 1/200 dilution for 2 h; 2 × 15-min washes, vector ABC staining as per kit directions; 2 × 15-min washes, 0.05% DAB). The characteristics of these antisera have been reported (12, 13). Staining for the lectin IB4 was performed as described (13) by using biotinylated IB4 (Sigma) and then the same DAB protocol as used above. For cell counts of CGRP, substance P, neurofilament, and IB4-labeled cells procedures were the same as described above for stereological counting, except that cell counts were done for cell tops with no immunohistochemical labeling and cell tops that were also labeled for one of the four markers of interest. At least 300 cells from each DRG with a visible nucleolus were counted.
To quantitate the regeneration distance after a crush injury, 100-μm longitudinal sections of sciatic nerve were immunostained with a previously described anti-GAP43 antibody (14) at a dilution of 1/50,000. GAP43 is rapidly expressed by regenerating axons and is also a marker for nonmyelin-forming Schwann cells (14). At this dilution, no GAP43 staining was detected in the intact, uninjured nerve while regenerating axons could be readily identified after nerve injury. The length of axonal regeneration was quantified by image analysis using NIH image (Scion, Frederick, MD).
Cellular apoptosis was measured by using age-matched mutant and wild-type mice. The spinal columns were removed and fixed by immersion in 4% paraformaldehyde overnight at 4°C. Tissue was cryopreserved in 20% sucrose, sectioned at 12 mm on a cryostat, and processed with terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL) to label apoptotic cells (Boehringer Mannheim). Sections were counterstained by using hematoxylin and mounted. The entire DRG was sectioned, and the total number of TUNEL-positive cells were manually counted. Cells were only counted as being TUNEL-positive if they were small, pyknotic, and brown. Cells at the edge of each section were not counted to ensure against bias by nonspecific staining. Appropriate controls were included in every experiment. A minimum of 10 DRG/animal were quantified.
DRG Culture.
DRGs from the lumbar, cervical, and thoracic region of 8-week-old adult mice were removed aseptically and collected in DMEM/F12 medium. Ganglia were enzymatically treated with collagenase for 1 h at 37°C, washed, and digested with trypsin EDTA for 10 min at 37°C. After washing, ganglia were mechanically dissociated in medium containing trypsin inhibitor. After centrifugation, cells were resuspended in DMEM/F12 medium supplemented with 5% horse serum, 1 mM glutamine, and 10 ng/ml gentamycin. Cells were preplated overnight on 6-well plates coated with 0.5 mg/ml polyornithine to remove glial cells. Medium then was removed, and the neurons were squirted off the surface with fresh medium. After centrifugation, cells were plated on 24-well plates treated with 0.5 mg/ml polyornithine and 5 μg/ml laminin and maintained at 37°C in a humidified incubator for a period of 8 or 24 h. Neurite length cannot be accurately assessed after 24 h in culture because the processes are so long they all merge into each other even when plating at very low cell density. Cultures were fixed with 4% paraformaldehyde for 20 min at room temperature. Neurite length was visualized by phase-contrast microscopy and quantified by image analysis using NIH image (Scion).
Results
A Subpopulation of Nociceptive Neurons Are Lost by Increased Cell Death in the DRG of Galanin Mutant Mice.
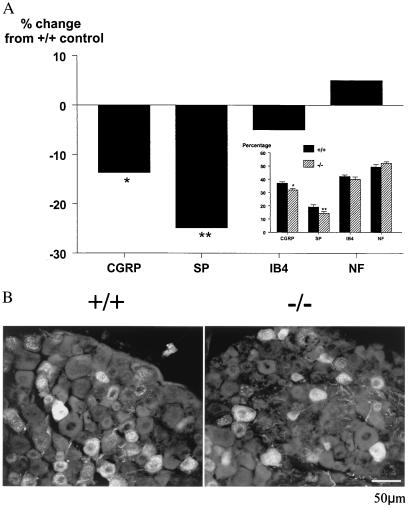
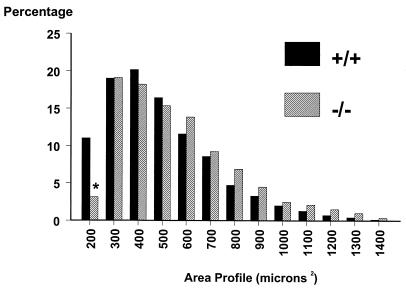
To determine whether the absence of galanin throughout development affects the final number of neurons in the DRG, stereological counting of L4 and L5 DRG neurons in intact adult animals was performed. Results demonstrate a statistically significant decrease of 13% in the number of neurons in mutant animals compared with wild-type controls (8,220 ± 216 vs. 9,460 ± 412 mutant and wild type, respectively, P < 0.01, n = 8). It should be noted that the L4 and L5 DRG neurons were separately counted and then compared; the above differences were identical for both sets of DRG (data not shown). To identify whether specific subpopulations of DRG neurons are affected in the mutant animals, we measured the percentage of cells that express substance P, CGRP, markers of small C-fiber neurons, and the lectin IB4 and neurofilament, which mark nonpeptidergic C fibers and A fibers, respectively. Significant decreases of 24% and 15% were noted in substance P and CGRP, respectively in the mutant animals compared with wild-type controls (Fig. 1 A and B), whereas no differences were noted in the percentage of cells expressing IB4 and neurofilament, suggesting that small-diameter peptidergic nociceptive neurons are preferentially lost. To confirm this hypothesis, when the cross sectional profiles of DRG neurons were studied there was a significant decrease in the percentage of the smallest neurons (Fig. 2). Taken together, these studies confirm that much of the cell loss in the DRG involves small peptidergic neurons, suggesting that galanin expression is essential for the developmental survival of a subset of neurons that are most likely to be nociceptors.
Figure 1.
(A) The percentage of cells histochemically expressing the markers CGRP, substance P (SP), IB4, and neurofilament (NF) in adult wild-type and mutant DRG. (Inset) Graph represents absolute values rather than percentage change for each marker. Significant deficits were observed in the percentage of CGRP- and substance P- expressing neurons, whereas the percentages of cells expressing IB4 and NF were unchanged (t test; *, P < 0.05; **, P < 0.01, n = 6). It should be noted that these markers are not completely exclusive of each other and in some cases may colocalize in single neurons. (B) Representative photomicrograph demonstrating fewer substance P-positive cells in the DRG from mutant (Right) as compared with wild-type (Left) animals.
Figure 2.
Cell size distributions for DRG neurons from wild-type and mutant animals. Cell area was determined in cross sectional profiles of DRG neurons showing a visible nucleolus, with at least 100 cells measured per DRG. No differences were noted between the two genotypes other than in the smallest diameter neurons (*, P < 0.05, n = 8).
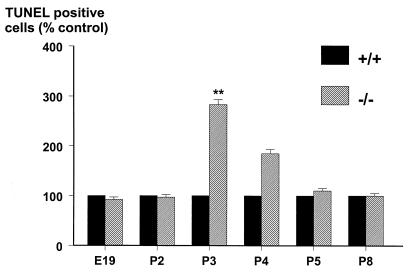
We hypothesized that the reduced populations of DRG neurons in the mutant mice might be caused by an increased loss of cells by apoptosis. To address this and to define the time point during development when the cell loss in the DRG occurred, we visualized apoptosis in the DRG of age-matched wild-type and mutant embryos or neonates by using the TUNEL method. No differences were noted in the number of TUNEL-positive cells at embryonic day (E)19 or at P2 in age-matched wild-type and mutant animals (Fig. 3). In contrast, a 2.8- and 2.6-fold increase in the number of TUNEL-positive cells was noted at P3 and P4, respectively, in the mutants that were not present at P5 or thereafter (Fig. 3), suggesting that there is a concerted wave of cell death that occurs around P3/4, because of the absence of galanin.
Figure 3.
The number of TUNEL-positive cells in DRG sections from mutant animals at various time points during embryonic and postnatal development were manually counted. The results are expressed as a percentage of age-matched wild-type controls demonstrating a wave of apoptosis in the mutants at P3 and P4 that was not seen at earlier or later time points (t test; **, P < 0.01, n = 5).
Axonal Outgrowth and Regeneration Are Reduced in DRG Sensory Neurons of Galanin Mutant Mice.
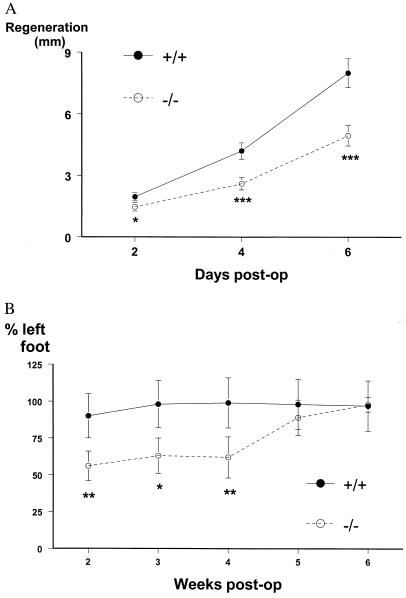
To study whether galanin plays a growth-promoting role in the adult, the ability of damaged axons to regenerate after a crush injury was studied in wild-type and mutant animals. The rate of axonal regeneration of the sciatic nerve after a crush injury was measured in three separate assays. First, regeneration in the week after a crush injury was quantified by using the “sensory pinch test.” In this test, the distance from the nerve crush site to the foremost regenerating sensory axons is determined by pinching consecutive segments of the nerve in a distal to proximal direction until a contraction of the abdominal muscles in the back is observed (15). This test depends on retrograde conduction along sensory neurons and is independent of sciatic nerve motor function. The actual regeneration distance is measured by using a graticule viewed under a dissector microscope to quantify the distance from the original crush site marked with charcoal and the pinch site that elicited an abdominal response. The test cannot be used once the regeneration front has extended beyond the bifurcation/trifurcation of the sciatic nerve (after which time the regeneration distance cannot be accurately measured) and thus can only be used in the first week after the crush injury. The regeneration distance at 2, 4, and 6 days after nerve crush showed a statistically significant reduction of 30–40% in mutants compared with wild-type mice (Fig. 4A). Secondly, regenerating growth cones were visualized by immunocytochemical staining for GAP43 (14) on longitudinal sections of sciatic nerve, and the regeneration distance from the crush site was quantified by image analysis. Results demonstrate a 34% reduction in the regeneration distance in mutants on day 1 postcrush (725 μm vs. 475 μm, P < 0.01 wild type and mutant, respectively, n = 6) and a 36% reduction on day 2 (1,180 μm vs. 750 μm, P < 0.01 wild type and mutant, respectively, n = 6). Thereafter, the up-regulation of GAP43 expression in Schwann cells made the regenerating growth cones difficult to visualize accurately (14). Finally, to determine whether the reduced rate of regeneration in galanin-deficient mice after a crush injury affects long-term functional recovery, we also tested a behavioral correlate of regeneration by using the toe spreading index (16). Rodents spread the toes on their hind feet on contact with a solid surface, a reflex that requires sensory innervation. The ability to do this therefore is reduced after a crush injury and returns to normal once sensory axon reinnervation occurs. The actual distance between the first and fifth toe of the hind paw varies between 3 and 8 mm. This distance is measured by constructing a perspex corridor 1 m in length, which is placed on plain white paper. The hind paws are dipped in blue food coloring, and the animal is allowed to walk down the corridor on the paper. In this way a minimum of 20 hind-paw prints are obtained and the distance between the first and fifth toe of the hind paw is measured. For each animal and at each time point, the toe spread of the injured paw is expressed as a percentage of the uninjured paw. The injured paw reliably begins to spread at 2 weeks when axons begin to reinnervate the foot muscles, explaining why the first time point on the graph is 2 weeks. Although toe spreading in wild-type mice returned to normal within 3 weeks of sciatic nerve crush, considerable functional deficits persisted in the mutant mice (Fig. 4B). Functional regeneration was still incomplete at 4 weeks in the mutant mice but had normalized by 5 weeks after the crush injury.
Figure 4.
(A) Regenerative abilities of sensory axons in the sciatic nerve after a crush injury were measured by using the pinch test. Mutant mice showed statistically reduced rates of regeneration compared with the wild-type control group at all time points (t test; *, P < 0.05; ***, P < 0.001, n = 8). Data are presented as regeneration distance (mm) ± SEM. (B) Long-term functional recovery after a crush injury to the sciatic nerve measured by using the toe spreading index. Mutant mice showed statistically reduced rates of regeneration compared with the wild-type control group at weeks 2–4 (t test; *, P < 0.05; **, P < 0.01, n = 8). Data are presented as percentage recovery compared with the uninjured foot ± SEM.
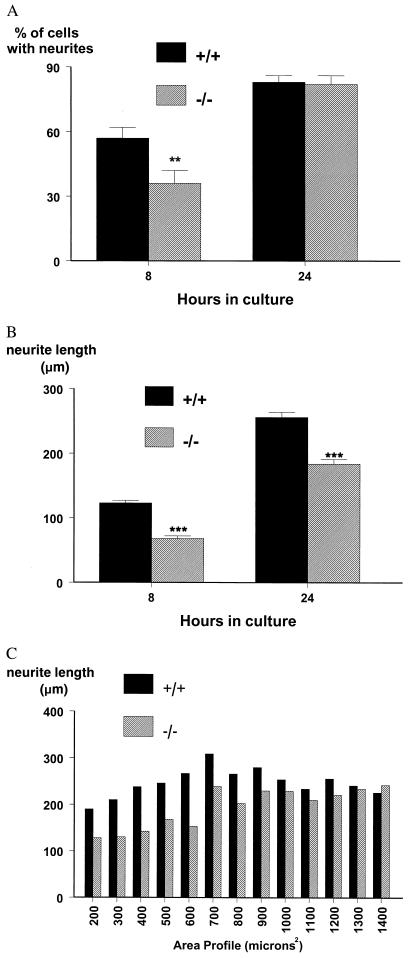
Having defined deficits in the rate of regeneration in vivo, we then asked whether the ability of dispersed DRG cells to extend neurites in culture also was impaired. Adult mutant and wild-type cells were studied in culture at 8 and 24 h, and the percentage of cells extending neurites, as defined by outgrowth of a process greater than two cell diameters, and the length of the neuritic processes were measured. The number of mutant cells producing neurites was reduced by a third at 8 h compared with wild-type controls, but was equal to that observed in the wild types by 24 h in culture (Fig. 5A), indicating an increased lag time during the initiation phase of regeneration. Highly significant deficits in neurite length were observed at both time points (45% and 28% at 8 and 24 h, respectively) in cells obtained from adult mutant animals, compared with those from wild-type controls (Fig. 5B). To further define whether a specific subset of cells in the mutant animals was affected by the absence of galanin, neurite length was plotted as a function of area profile after 24 h in culture. It is clear (Fig. 5C) that mutant cells in the 200- to 600-μm range produce considerably shorter neurites than wild-type control cells of the same area profile. In contrast, equal neurite outgrowth from larger cells (≥700 μm) was noted from both genotypes.
Figure 5.
(A) The percentage of cells bearing neurites at 8 and 24 h in culture from wild-type and mutant animals and (B) length of neurite outgrowth at both time points. In both cases, statistically significant deficits are noted in the mutant animals (t test; **, P < 0.01; ***, P < 0.001, n = 5). Data are presented as percentage of cells bearing neurites or mean neurite length ± SEM. (C) Neurite length as a function of area profile at 24 h in culture for both genotypes, demonstrating that small cells produce considerably shorter neurites in mutants compared with wild-type controls (n = 5).
Discussion
Peripheral nerve damage in mammals induces major and long-lasting changes in the expression of numerous secreted ligands and their receptors in primary sensory neurons (3), including the neurotrophins, members of the transforming growth factor type β superfamily, cytokines, and a number of neuropeptides. Extensive research using mouse molecular genetics has demonstrated that the developmental survival and differentiation of primary sensory neurons are principally regulated by the neurotrophins (17–19) and glial cell line-derived neurotrophic factor-related ligands (13, 20). In the adult, exogenously applied neurotrophins accelerate regeneration, remyelination, and recovery of behavioral and electrophysiological deficits after nerve injury (21). Further, overexpression of the pan-neurotrophin PNT-1 in adult transgenic animals has been demonstrated to accelerate the regeneration of sensory and motor axons after peripheral nerve damage (22). To date there is little evidence that neuropeptides are important for either cell survival or regeneration of sensory neurons after injury.
Because most DRG neurons express galanin during development and its marked up-regulation after injury and apparent axonal transport, we hypothesized that galanin may play a role in the development and regeneration of sensory neurons. The data presented here substantiate such a hypothesis, demonstrating that galanin plays a critical role in the survival of a subset of DRG sensory neurons in the early postnatal period. Galanin would appear to play a postnatal role that is analogous to the nerve growth factor-TrkA interaction during late embryonic development, where the majority of cell death in the DRG occurs in a wave that starts at E15 and peaks at E17–19. Previous stereological data also has demonstrated a second smaller wave of apoptosis that occurs at P3 and is associated with a 16% decrease in the total DRG cell number (23). As with the nerve growth factor knockout animals, the cell loss by apoptosis that usually occurs in the DRG is magnified by the absence of galanin. The above data would indicate that galanin exerts direct paracrine effects on survival within the DRG in the early postnatal period. Alternatively, it is possible that delayed neurite outgrowth and the subsequent loss of target-derived neurotrophic support may underlie the increased cell death at P3/4, although this is less likely because most sensory axons have reached their peripheral targets by birth. In either case, the loss of a subset of small unmyelinated neurons that are likely to be nociceptors in the adult DRG may provide, in part, an explanation for our most recent data (10), demonstrating that mutant animals show a decrease in chronic neuropathic pain behavior after nerve injury. Further, the finding that the neuropeptide galanin plays a developmental role to the DRG is not specific to the peripheral nervous system. Galanin is also essential for the developmental survival of a third of the cholinergic neurons in the basal forebrain. The loss of a subset of cholinergic septo-hippocampal neurons is associated with marked deficits in performance in the Morris water maze and in the induction of long-term potentiation in the mutant animals (24).
The involvement of galanin in the survival of a subset of DRG neurons is likely to be mediated by the activation of galanin receptors. One therefore would expect that the neurons that depend on galanin for their survival would express one or more of the galanin receptor subtypes at some point in their development. Three G protein-coupled galanin receptor subtypes have been identified to date. The first subtype (25) (GALR1) has a more restricted DRG expression pattern than its ligand during development (2). Two additional galanin receptor subtypes now have been identified, designated GALR2 (26–28) and GALR3 (29), with 38% and 36% homology, respectively, to the previously cloned receptor GALR1. Binding of galanin to GALR1 and GALR3 inhibits adenylyl cyclase (25, 29, 30), whereas binding to GALR2 stimulates phospholipase C activity and increases intracellular inositol triphosphate turnover (26–28), raising the possibility that the receptor subtypes function in divergent molecular cascades and may play differing roles relating to growth and cell survival. A number of groups recently have demonstrated that differing subpopulations of DRG and motor neurons express these receptor subtypes and that their levels and patterns of expression change after injury (31–34). GALR1 expressing neurons in the DRG are larger than those that express GALR2, whereas only 20% of cells express both receptor subtypes (31, 32, 35). GALR3 also is expressed in the DRG (36), but the phenotypes of the receptor-bearing neurons are as yet unknown. Our data imply that galanin primarily acts, both developmentally and in the adult, on the small-sized neurons that would be expected to express GALR2. Because antisera to the cloned receptor subtypes are at present unavailable, expression studies at the protein level in wild-type and mutant animals during development and in the adult are not yet possible.
In addition to the developmental cell-survival role described above, galanin appears to play a neuritogenic role after injury in the adult, as functional and anatomical measures of regeneration are markedly impaired in the mutant animals. There are clear similarities between the in vivo and in vitro data, as the rate of neuronal outgrowth is reduced 30–40% both during regeneration and in the first 24 h of culture. Further, the absence of galanin does not appear to affect the number of cells that are capable of extending neurites in culture, merely the rate at which outgrowth occurs (see Fig. 5 A and B). These data are thus analogous to the whole-animal long-term regeneration data (Fig. 4B) where complete regeneration does occur, albeit at a much reduced rate. At present it is not possible to state the relationship between the developmental loss of a subset of small peptidergic neurons and the reduced rate of growth of axons from small diameter neurons in the adult. Galanin may be acting on differing subsets of neurons during development and in the adult after injury, possibly mediated by differing levels of expression or combinations of receptor subtypes.
The precise molecular basis for the up-regulation of galanin expression after injury is unknown. Recently, several lines of evidence indicate that the cytokines leukemia inhibitory factor (LIF) and IL-6 regulate galanin gene expression in the DRG after peripheral nerve damage. (a) Injection of LIF or IL-6 into the sciatic nerve significantly increases galanin expression in the DRG (37, 38). (b) Conversely, axotomy-induced up-regulation of galanin is markedly attenuated in IL-6 (37) and LIF knockout mice (39–41). (c) Both LIF and IL-6 bind to the gp130 receptor and initiate signal transduction through Janus kinase-signal transducers and activators of transcription (STAT) pathways. Targeted disruption of the gp130 receptor causes a developmental loss of approximately 15% of DRG neurons (42), similar in magnitude to that observed in the galanin mutants. (d) The rat (43) and mouse (D.W., unpublished data) galanin genes have at least one STAT binding site ≈2.5 kb upstream of the transcriptional start site.
Taken together, these findings imply that the cytokines LIF and IL-6 (acting through the gp130 receptor) and galanin function in a molecular cascade, mediating injury-induced regeneration and minimizing pathological nociceptive responses. Peripheral nerve damage up-regulates the expression of the cytokines LIF (44) and IL-6 (45) by Schwann cells within the nerve, and in the case of IL-6, in the DRG neurons themselves (37). These changes markedly increase the levels of galanin in the DRG (37, 38). Rising levels of galanin in sensory neurons contribute to the initiation and maintenance of axonal regeneration in the injured neurons, leading to functional recovery and restoration of appropriate nociception. In support of this molecular cascade, IL-6 knockout mice demonstrate striking similarities to the galanin mutants in terms of deficits in peripheral nerve regeneration after a crush of the sciatic nerve and an attenuation of neuropathic pain (37, 46). These findings, therefore, have important implications for the understanding, and potential therapeutic treatment, of peripheral nerve regeneration in response to trauma and sensory neuropathies.
Acknowledgments
This work was supported by The Medical Research Council and The Wellcome Trust. V.R.K. was supported by St. Thomas's Hospital Research Endowments.
Abbreviations
- DRG
dorsal root ganglion
- Pn
postnatal day
- En
embryonic day
- CGRP
calcitonin gene-related peptide
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling
- LIF
leukemia inhibitory factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210221897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210221897
References
- 1.Hokfelt T, Wiesenfeld H Z, Villar M, Melander T. Neurosci Lett. 1987;83:217–220. doi: 10.1016/0304-3940(87)90088-7. [DOI] [PubMed] [Google Scholar]
- 2.Xu Z Q, Shi T J, Hokfelt T. Proc Natl Acad Sci USA. 1996;93:14901–14905. doi: 10.1073/pnas.93.25.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hokfelt T, Zhang X, Wiesenfeld H Z. Trends Neurosci. 1994;17:22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Verge V M, Wiesenfeld H Z, Piehl F, Hokfelt T. Exp Brain Res. 1993;93:450–461. doi: 10.1007/BF00229360. [DOI] [PubMed] [Google Scholar]
- 5.Mohney R P, Siegel R E, Zigmond R E. J Neurobiol. 1994;25:108–118. doi: 10.1002/neu.480250203. [DOI] [PubMed] [Google Scholar]
- 6.Villar M J, Cortes R, Theodorsson E, Wiesenfeld H Z, Schalling M, Fahrenkrug J, Emson P C, Hokfelt T. Neuroscience. 1989;33:587–604. doi: 10.1016/0306-4522(89)90411-9. [DOI] [PubMed] [Google Scholar]
- 7.Villar M J, Wiesenfeld H Z, Xu X J, Theodorsson E, Emson P C, Hokfelt T. Exp Neurol. 1991;112:29–39. doi: 10.1016/0014-4886(91)90111-o. [DOI] [PubMed] [Google Scholar]
- 8.Shadiack A M, Zigmond R E. Neuropeptides. 1998;32:257–264. doi: 10.1016/s0143-4179(98)90045-2. [DOI] [PubMed] [Google Scholar]
- 9.Wynick D, Small C J, Bacon A, Holmes F E, Norman M, Ormandy C J, Kilic E, Kerr N C H, Ghatei M, Talamantes F, et al. Proc Natl Acad Sci USA. 1998;95:12671–12676. doi: 10.1073/pnas.95.21.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr B J, Cafferty W B J, Gupta Y K, Bacon A, Wynick D, McMahon S B, Thompson S W N. Eur J Neurosci. 2000;12:793–802. doi: 10.1046/j.1460-9568.2000.00967.x. [DOI] [PubMed] [Google Scholar]
- 11.Gundersen H J, Bagger P, Bendtsen T F, Evans S M, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J R, Pakkenberg B, et al. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 12.Baranowski A P, Priestley J V, McMahon S B. Neurosci Lett. 1994;168:197–200. doi: 10.1016/0304-3940(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 13.Bennett D L, Michael G J, Ramachandran N, Munson J B, Averill S, Yan Q, McMahon S B, Priestley J V. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis R, Stewart H J, Hall S M, Wilkin G P, Mirsky R, Jessen K R. J Cell Biol. 1992;116:1455–1464. doi: 10.1083/jcb.116.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutmann E, Guttmann L, Medawar P, Young J. J Exp Biol. 1942;19:14–44. [Google Scholar]
- 16.Hoogeveen J F, Van Der Kracht A H, Wondergem J, Gonzalez Gonzalez D, Haveman J. Neurotoxicology. 1993;14:1–7. [PubMed] [Google Scholar]
- 17.Lewin G R, Barde Y A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 18.Ibanez C F. Trends Biotechnol. 1995;13:217–227. doi: 10.1016/S0167-7799(00)88949-0. [DOI] [PubMed] [Google Scholar]
- 19.Crowley C, Spencer S D, Nishimura M C, Chen K S, Pittsmeek S, Armanini M P, Ling L H, McMahon S B, Shelton D L, Levinson A D, et al. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 20.Munson J B, McMahon S B. Eur J Neurosci. 1997;9:1126–1129. doi: 10.1111/j.1460-9568.1997.tb01465.x. [DOI] [PubMed] [Google Scholar]
- 21.Munson J B, Shelton D L, McMahon S B. J Neurosci. 1997;17:470–476. doi: 10.1523/JNEUROSCI.17-01-00470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funakoshi H, Risling M, Carlstedt T, Lendahl U, Timmusk T, Metsis M, Yamamoto Y, Ibanez C F. Proc Natl Acad Sci USA. 1998;95:5269–5274. doi: 10.1073/pnas.95.9.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coggeshall R E, Pover C M, Fitzgerald M. Brain Res Dev Brain Res. 1994;82:193–212. doi: 10.1016/0165-3806(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 24.O'Meara G, Coumis U, Shuang Y M, Kehr J, Mahoney S, Bacon A, Allen S J, Holmes F E, Kahl U, Wang F H, et al. Proc Natl Acad Sci USA. 2000;97:11569–11574. doi: 10.1073/pnas.210254597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habert Ortoli E, Amiranoff B, Loquet I, Laburthe M, Mayaux J F. Proc Natl Acad Sci USA. 1994;91:9780–9783. doi: 10.1073/pnas.91.21.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fathi Z, Cunningham A M, Iben L G, Battaglino P B, Ward S A, Nichol K A, Pine K A, Wang J C, Goldstein M E, Iismaa T P, et al. Mol Brain Res. 1997;51:49–59. doi: 10.1016/s0169-328x(97)00210-6. [DOI] [PubMed] [Google Scholar]
- 27.Howard A D, Tan C, Shiao L L, Palyha O C, McKee K K, Weinberg D H, Feighner S D, Cascieri M A, Smith R G, Van der Ploeg L H, et al. FEBS Lett. 1997;405:285–290. doi: 10.1016/s0014-5793(97)00196-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Hashemi T, He C, Strader C, Bayne M. Mol Pharmacol. 1997;52:337–343. doi: 10.1124/mol.52.3.337. [DOI] [PubMed] [Google Scholar]
- 29.Wang S K, He C G, Hashemi T, Bayne M. J Biol Chem. 1997;272:31949–31952. doi: 10.1074/jbc.272.51.31949. [DOI] [PubMed] [Google Scholar]
- 30.Smith K E, Walker M W, Artymyshyn R, Bard J, Borowsky B, Tamm J A, Yao W J, Vaysse P J, Branchek T A, Gerald C, et al. J Biol Chem. 1998;273:23321–23326. doi: 10.1074/jbc.273.36.23321. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell D, Ahmad S, Wahlestedt C, Walker P. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- 32.Xu Z Q, Shi T J, Landry M, Hokfelt T. NeuroReport. 1996;8:237–242. doi: 10.1097/00001756-199612200-00048. [DOI] [PubMed] [Google Scholar]
- 33.Ji R R, Zhang Q, Bedecs K, Arvidsson J, Zhang X, Xu X J, Wiesenfeld H Z, Bartfai T, Hokfelt T. Proc Natl Acad Sci USA. 1994;91:12540–12543. doi: 10.1073/pnas.91.26.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burazin T C D, Gundlach A L. J Neurochem. 1998;71:879–882. doi: 10.1046/j.1471-4159.1998.71020879.x. [DOI] [PubMed] [Google Scholar]
- 35.Sten-Shi T J, Zhang X, Holmberg K, Xu Z Q, Hokfelt T. Neurosci Lett. 1997;237:57–60. doi: 10.1016/s0304-3940(97)00805-7. [DOI] [PubMed] [Google Scholar]
- 36.Waters S M, Krause J E. Neuroscience. 2000;95:265–271. doi: 10.1016/s0306-4522(99)00407-8. [DOI] [PubMed] [Google Scholar]
- 37.Murphy P G, Ramer M S, Borthwick L, Gauldie J, Richardson P M, Bisby M A. Eur J Neurosci. 1999;11:2243–2253. doi: 10.1046/j.1460-9568.1999.00641.x. [DOI] [PubMed] [Google Scholar]
- 38.Thompson S W, Priestley J V, Southall A. Neuroscience. 1998;84:1247–1255. doi: 10.1016/s0306-4522(97)00553-8. [DOI] [PubMed] [Google Scholar]
- 39.Corness J, Shi T J, Xu Z Q, Brulet P, Hokfelt T. Exp Brain Res. 1996;112:79–88. doi: 10.1007/BF00227180. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Zigmond R E. Eur J Neurosci. 1996;8:2213–2220. doi: 10.1111/j.1460-9568.1996.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 41.Kerekes N, Landry M, Hokfelt T. Neuroscience. 1999;89:1123–1134. doi: 10.1016/s0306-4522(98)00405-9. [DOI] [PubMed] [Google Scholar]
- 42.Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Yoshida K, Kishimoto T, Sendtner M, Taga T. J Neurosci. 1999;19:5429–5434. doi: 10.1523/JNEUROSCI.19-13-05429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corness J, Burbach J P H, Hokfelt T. Mol Brain Res. 1997;47:11–23. doi: 10.1016/s0169-328x(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 44.Curtis R, Scherer S S, Somogyi R, Adryan K M, Ip N Y, Zhu Y, Lindsay R M, DiStefano P S. Neuron. 1994;12:191–204. doi: 10.1016/0896-6273(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 45.Bolin L M, Verity A N, Silver J E, Shooter E M, Abrams J S. J Neurochem. 1995;64:850–858. doi: 10.1046/j.1471-4159.1995.64020850.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhong J, Dietzel I D, Wahle P, Kopf M, Heumann R. J Neurosci. 1999;19:4305–4313. doi: 10.1523/JNEUROSCI.19-11-04305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]