Abstract
AIMS—To investigate the ultrastructural localisation of proteoglycans (PG), βig-h3 (keratoepithelin), tenascin-C (TN-C)), fibrillin, and fibronectin in bullous keratopathy (BK) corneas. METHODS—Five corneas from cases of pseudophakic bullous keratopathy (BK) were examined by electron microscopy. PG were demonstrated using cuprolinic blue, and the proteins βig-h3, TN-C, fibrillin, and fibronectin were immunolocalised with rabbit anti-βig-h3, mouse anti-TN-C (BC10 and TN2), mouse anti-fibrillin-1 (MAB2502), mouse anti-fibrillin (MAB1919), and rabbit anti-fibronectin by using a standard immunogold technique. RESULTS—Epithelial cells contained numerous vacuoles. Epithelial folds and large, electron lucent subepithelial bullae were present. Basal lamina was thickened and traversed by disrupted anchoring filaments. In the stroma, interfibrillar collagen spacing was increased and abnormally large PG were present. Descemet's membrane (DM) contained lucent spaces in which there were small filaments. Keratocyte and endothelial cells contained melanin granules. A posterior collagenous layer (PCL) contained numerous microfilaments and wide spacing collagen fibres with a periodicity of 100 nm. Large quantities of abnormal PG were observed at the endothelial face of the PCL. Very strong labelling with βig-h3 antibody was observed in the basement membrane, Bowman's layer, stroma, DM, and PCL, but not in keratocytes and endothelial cells. Strong labelling with BC10 and TN2 was seen below the epithelium, in electron lucent spaces where the hemidesmosomes were absent, in the fibrotic pannus, in parts of Bowman's layer, the stroma, and Descemet's membrane. Labelling with BC10 was stronger and more evenly distributed than with TN2. Fibrillin-1 (MAB2502) and fibrillin (MAB1919) labelling was similar to TN-C labelling. Fibrillin (MAB1919) labelling was stronger than fibrillin-1 (MAB2502) labelling. CONCLUSIONS—Immunoelectron microscopy showed precise labelling of proteins at both the cellular and the subcellular level. Expression of proteins βig-h3, TN-C, fibrillin, and fibronectin was highly increased compared with normal cornea. In the oedematous stroma, increased collagen fibril separation may facilitate a wider distribution of some soluble proteins, such as βig-h3, throughout stroma. The modified expression of the proteins studied in these cases of BK may be regarded as part of an injury response.
Full Text
The Full Text of this article is available as a PDF (538.7 KB).
Figure 1 .
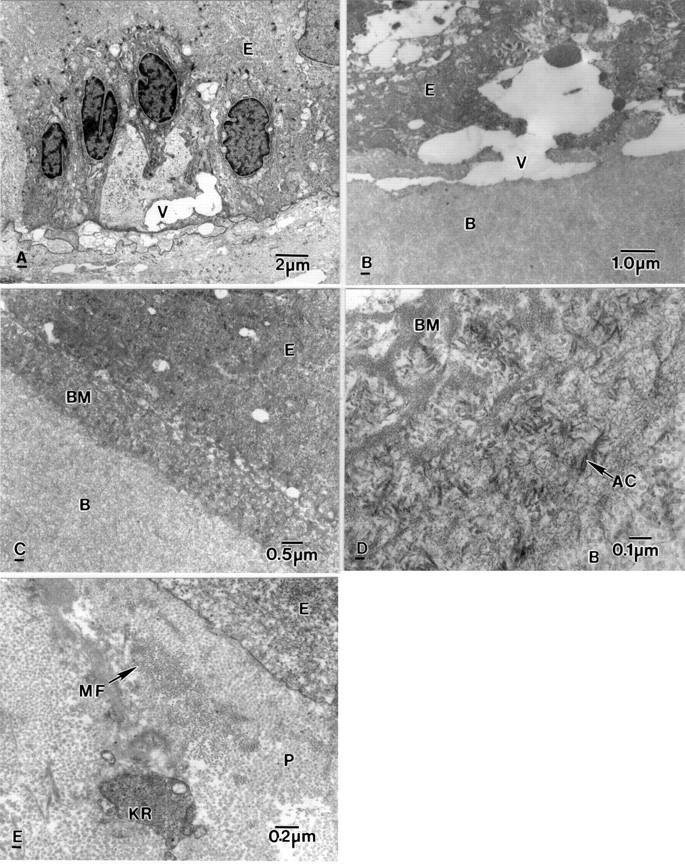
Electron micrographs showing ultrastructural alteration in a bullous keratopathy cornea. (A) Electron lucent vacuole (V) in a degenerate basal epithelial cell (E) and vacuoles in the subepithelial region. (B) Subepithelial vacuole (V) between an epithelial cell (E) and Bowman's layer (B). (C) Thick basement membrane (BM) between epithelial cells (E) and Bowman's layer (B). (D) Basement membrane (BM) containing anchoring filaments (AC) above Bowman's layer (B). (E) Subepithelial pannus (P) containing microfilaments (MF) and rounded keratocytes (KR).
Figure 2 .
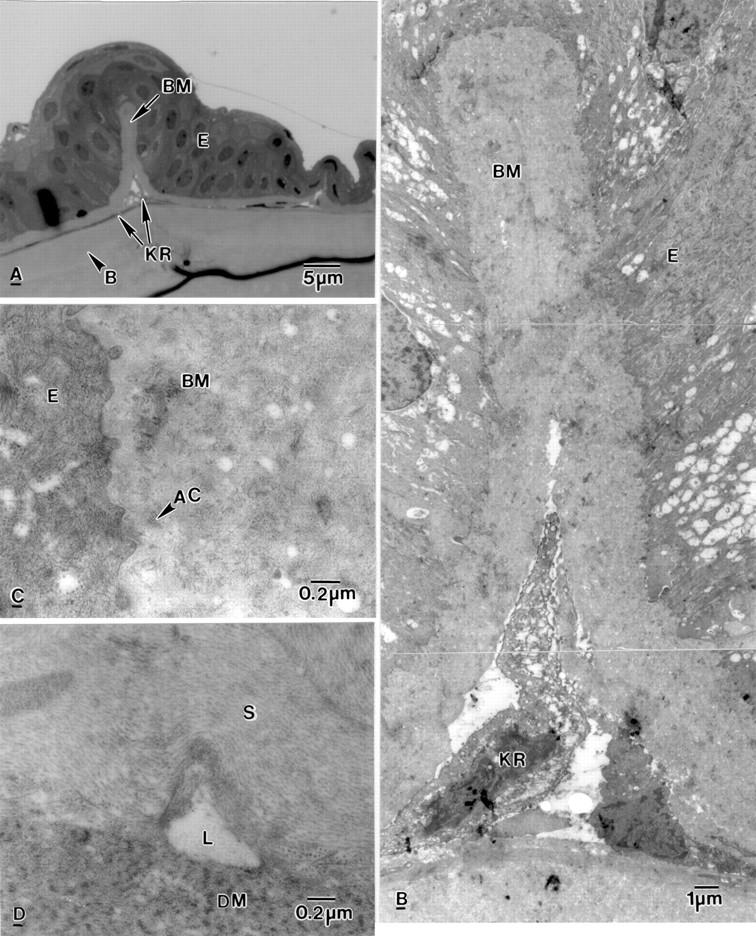
Light and electron micrograph of bullous keratopathy cornea. (A) Epithelial fold containing basement membrane material (BM). Keratocytes (KR) are arranged in a line above Bowman's layer (B) and the anterior stroma. (B) High magnification of the epithelial fold shown in (A). Keratocytes (KR) are present in the epithelial fold which contains basement membrane material (BM). (C) Part of an epithelial fold showing disrupted anchoring filaments (AC) in basement membrane material (BM). (D) Electron lucent lake (L) between the stroma (S) and Descemet's membrane (DM).
Figure 3 .
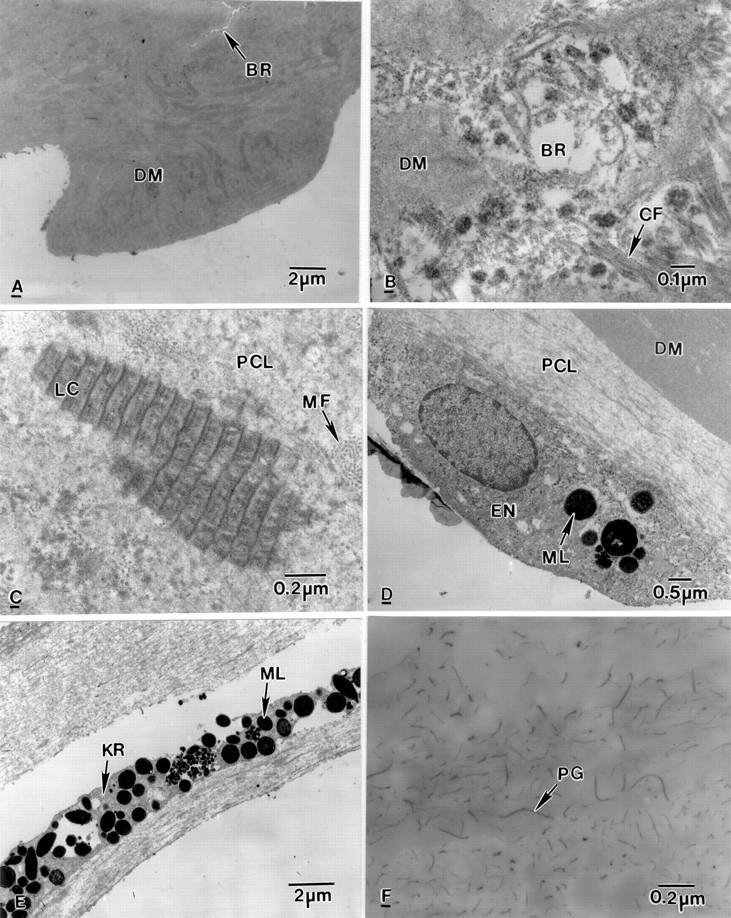
Electron micrographs of the posterior part of bullous keratopathy corneas. (A) Part of Descemet's membrane (DM) with a break (BR). (B) Collagen fibres (CF) and electron dense material in a break (BR) present in Descemet's membrane (DM). (C) Posterior collagenous layer (PCL) containing wide spacing collagen (LC) and microfilaments (MF). (D) Below the PCL, an endothelial cell (EN) containing melanin granules (ML). (E) A degenerate keratocyte (KR) containing melanin granules (ML). (F) Part of anterior stroma containing abnormal proteoglycans (PG).
Figure 4 .
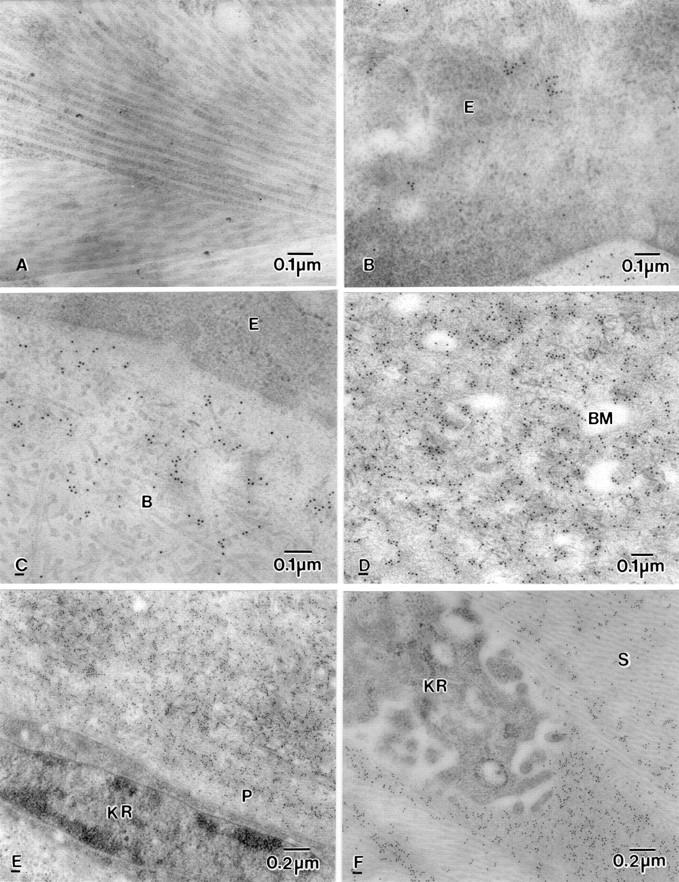
Electron micrograph showing labelling of βig-h3 in BK corneas. (A) Control for immunoreaction. (B) Clumped labelling in epithelial cell (E). (C) Moderate clumped labelling in normal looking Bowman's layer (B). (D) Heavy labelling in basement membrane (BM) and around disrupted anchoring filaments. (E) Diffuse labelling on fibrotic pannus tissue (P) but not in the keratocyte (KR). (F) No labelling in the keratocyte but heavy labelling close to the keratocyte (KR) in the anterior stroma (S).
Figure 5 .
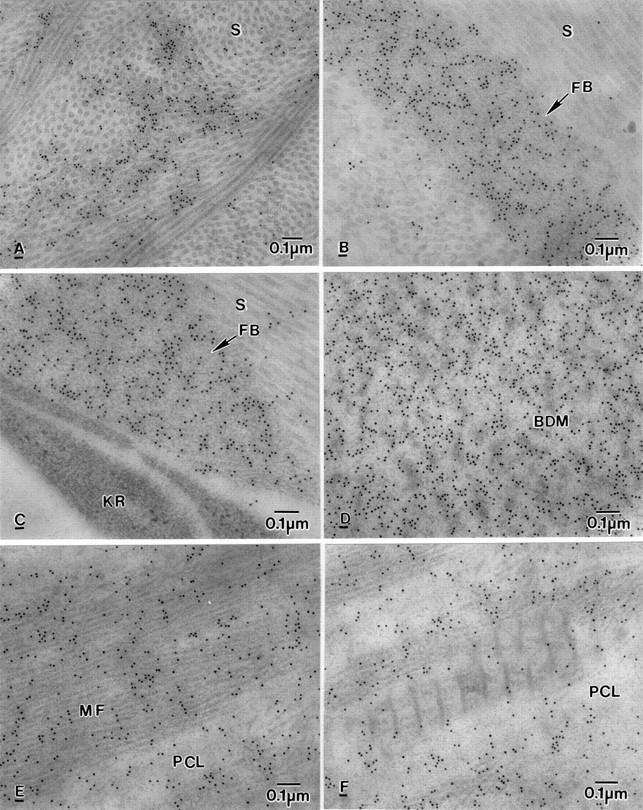
Electron micrograph showing labelling of βig-h3 in BK corneas. (A) Labelling on electron dense material present among collagen fibrils in the anterior stroma (S). (B) Labelling on fibrous (or fibrillar) material (FB) in the posterior stroma (S). (C) Labelling on fine fibrous (or fibrillar) material (FB) close to degenerate keratocyte (KR) but not in the keratocyte in the middle stroma (S). (D) Heavy labelling on banded Descemet's membrane (BDM). (E) Labelling on microfilaments (MF) in the PCL. (F) Labelling on electron dense material but not wide spacing collagen in the PCL.
Figure 6 .
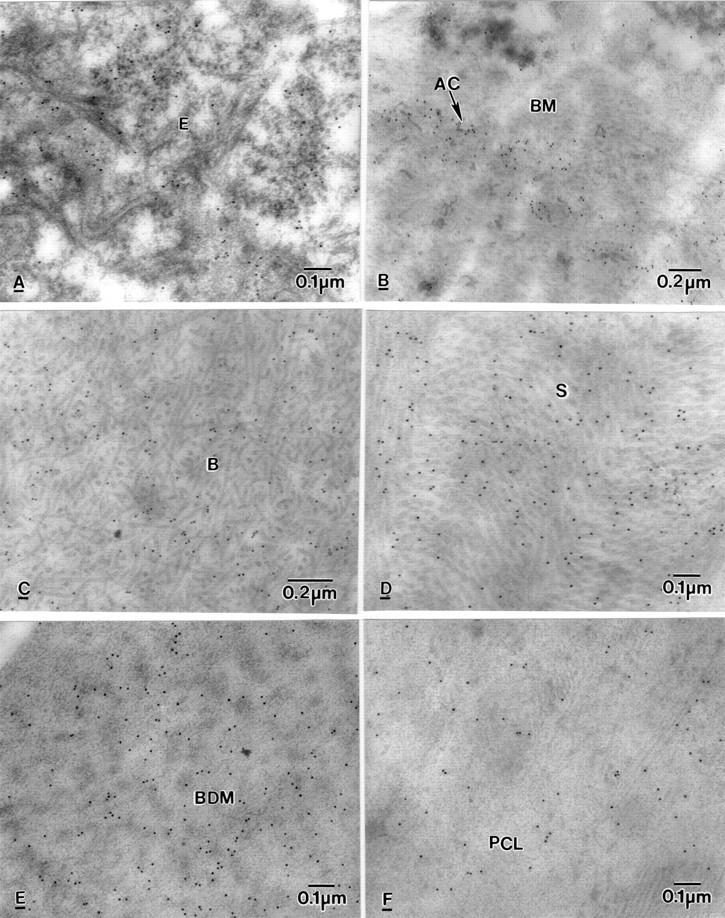
Electron micrographs of BK corneas showing labelling of BC10, in (A) epithelium (E), (B) Basement membrane (BM) and around anchoring filaments (AC), (C) Bowman's layer (B), (D) stroma (S), (E) banded Descemet's membrane (BDM), (F) posterior collagenous layer (PCL).
Figure 7 .
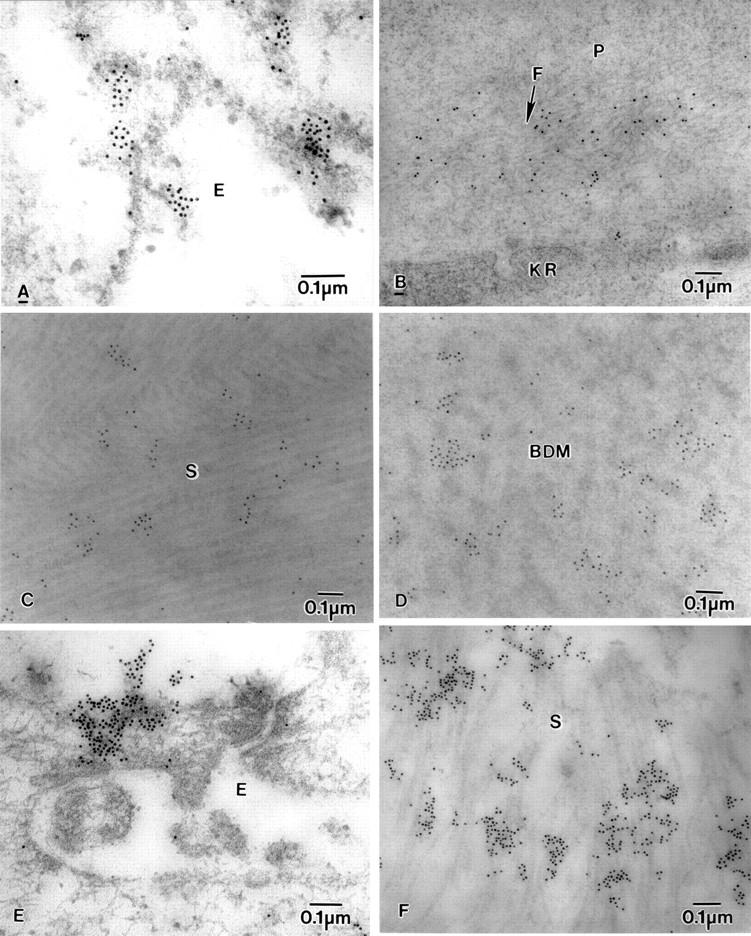
Electron micrograph of BK corneas showing labelling of fibrillin-1-MAB2502, fibrillin-MAB1919, and fibronectin. (A) Fibrillin-1-MAB2502 labelling in epithelial cell (E), (B) diffuse labelling of fibrillin-1-MAB2502 on microfibrils (F) near a degenerate keratocyte (KR) in the pannus (P). (C) fibrillin-MAB1919 labelling on collagen fibrils in stroma (S), (D) Fibrillin-MAB1919 labelling on banded Descemet's membrane (BDM). (E) Labelling of fibronectin on the surface of the epithelium (E), and (F) in the stroma (S).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amalric P. The choroid: from the architect gene to apoptosis. Lecture on acceptance of the Hermann Wacker Prize 1996. Graefes Arch Clin Exp Ophthalmol. 1997 Nov;235(11):675–683. doi: 10.1007/BF01880665. [DOI] [PubMed] [Google Scholar]
- Borsi L., Allemanni G., Gaggero B., Zardi L. Extracellular pH controls pre-mRNA alternative splicing of tenascin-C in normal, but not in malignantly transformed, cells. Int J Cancer. 1996 May 29;66(5):632–635. doi: 10.1002/(SICI)1097-0215(19960529)66:5<632::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Bristow J., Tee M. K., Gitelman S. E., Mellon S. H., Miller W. L. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol. 1993 Jul;122(1):265–278. doi: 10.1083/jcb.122.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnemolla B., Leprini A., Borsi L., Querzé G., Urbini S., Zardi L. Human tenascin-R. Complete primary structure, pre-mRNA alternative splicing and gene localization on chromosome 1q23-q24. J Biol Chem. 1996 Apr 5;271(14):8157–8160. doi: 10.1074/jbc.271.14.8157. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Hagios C., Matsumoto K. The tenascin gene family. Perspect Dev Neurobiol. 1994;2(1):3–7. [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Matsuoka Y., Hofer U., Spring J., Bernasconi C., Chiquet M. Tenascin variants: differential binding to fibronectin and distinct distribution in cell cultures and tissues. Cell Regul. 1991 Nov;2(11):927–938. doi: 10.1091/mbc.2.11.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle R. C., Jr, Laibson P. R., Arentsen J. J. Epithelial abnormalities in chronic corneal edema: a histopathological study. Trans Am Ophthalmol Soc. 1989;87:107–124. [PMC free article] [PubMed] [Google Scholar]
- El-Shabrawi Y., Kublin C. L., Cintron C. mRNA levels of alpha1(VI) collagen, alpha1(XII) collagen, and beta ig in rabbit cornea during normal development and healing. Invest Ophthalmol Vis Sci. 1998 Jan;39(1):36–44. [PubMed] [Google Scholar]
- Erickson H. P. Tenascin-C, tenascin-R and tenascin-X: a family of talented proteins in search of functions. Curr Opin Cell Biol. 1993 Oct;5(5):869–876. doi: 10.1016/0955-0674(93)90037-q. [DOI] [PubMed] [Google Scholar]
- Escribano J., Hernando N., Ghosh S., Crabb J., Coca-Prados M. cDNA from human ocular ciliary epithelium homologous to beta ig-h3 is preferentially expressed as an extracellular protein in the corneal epithelium. J Cell Physiol. 1994 Sep;160(3):511–521. doi: 10.1002/jcp.1041600314. [DOI] [PubMed] [Google Scholar]
- Gibson M. A., Hatzinikolas G., Kumaratilake J. S., Sandberg L. B., Nicholl J. K., Sutherland G. R., Cleary E. G. Further characterization of proteins associated with elastic fiber microfibrils including the molecular cloning of MAGP-2 (MP25) J Biol Chem. 1996 Jan 12;271(2):1096–1103. doi: 10.1074/jbc.271.2.1096. [DOI] [PubMed] [Google Scholar]
- Gulcher J. R., Alexakos M. J., Le Beau M. M., Lemons R. S., Stefansson K. Chromosomal localization of the human hexabrachion (tenascin) gene and evidence for recent reduplication within the gene. Genomics. 1990 Apr;6(4):616–622. doi: 10.1016/0888-7543(90)90495-g. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Noshiro M., Ohno S., Kawamoto T., Satakeda H., Akagawa Y., Nakashima K., Okimura A., Ishida H., Okamoto T. Characterization of a cartilage-derived 66-kDa protein (RGD-CAP/beta ig-h3) that binds to collagen. Biochim Biophys Acta. 1997 Mar 1;1355(3):303–314. doi: 10.1016/s0167-4889(96)00147-4. [DOI] [PubMed] [Google Scholar]
- Hirano K., Klintworth G. K., Zhan Q., Bennett K., Cintron C. Beta ig-h3 is synthesized by corneal epithelium and perhaps endotheliumin Fuchs' dystrophic corneas. Curr Eye Res. 1996 Sep;15(9):965–972. doi: 10.3109/02713689609017642. [DOI] [PubMed] [Google Scholar]
- Jones F. S., Hoffman S., Cunningham B. A., Edelman G. M. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing, and binding regions. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1905–1909. doi: 10.1073/pnas.86.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplony A., Zimmermann D. R., Fischer R. W., Imhof B. A., Odermatt B. F., Winterhalter K. H., Vaughan L. Tenascin Mr 220,000 isoform expression correlates with corneal cell migration. Development. 1991 Jun;112(2):605–614. doi: 10.1242/dev.112.2.605. [DOI] [PubMed] [Google Scholar]
- Kenney M. C., Chwa M. Abnormal extracellular matrix in corneas with pseudophakic bullous keratopathy. Cornea. 1990 Apr;9(2):115–121. [PubMed] [Google Scholar]
- Kenyon K. R., Van Horn D. L., Edelhauser H. F. Endothelial degeneration and posterior collagenous proliferation in aphakic bullous keratopathy. Am J Ophthalmol. 1978 Mar;85(3):329–336. doi: 10.1016/s0002-9394(14)77723-2. [DOI] [PubMed] [Google Scholar]
- LaFleur D. W., Fagin J. A., Forrester J. S., Rubin S. A., Sharifi B. G. Cloning and characterization of alternatively spliced isoforms of rat tenascin. Platelet-derived growth factor-BB markedly stimulates expression of spliced variants of tenascin mRNA in arterial smooth muscle cells. J Biol Chem. 1994 Aug 12;269(32):20757–20763. [PubMed] [Google Scholar]
- LeBaron R. G., Bezverkov K. I., Zimber M. P., Pavelec R., Skonier J., Purchio A. F. Beta IG-H3, a novel secretory protein inducible by transforming growth factor-beta, is present in normal skin and promotes the adhesion and spreading of dermal fibroblasts in vitro. J Invest Dermatol. 1995 May;104(5):844–849. doi: 10.1111/1523-1747.ep12607024. [DOI] [PubMed] [Google Scholar]
- Liu G. J., Okisaka S., Mizukawa A., Momose A. Histopathological study of pseudophakic bullous keratopathy developing after anterior chamber of iris-supported intraocular lens implantation. Jpn J Ophthalmol. 1993;37(4):414–425. [PubMed] [Google Scholar]
- Ljubimov A. V., Burgeson R. E., Butkowski R. J., Couchman J. R., Wu R. R., Ninomiya Y., Sado Y., Maguen E., Nesburn A. B., Kenney M. C. Extracellular matrix alterations in human corneas with bullous keratopathy. Invest Ophthalmol Vis Sci. 1996 May;37(6):997–1007. [PubMed] [Google Scholar]
- Ljubimov A. V., Saghizadeh M., Spirin K. S., Mecham R. P., Sakai L. Y., Kenney M. C. Increased expression of fibrillin-1 in human corneas with bullous keratopathy. Cornea. 1998 May;17(3):309–314. [PubMed] [Google Scholar]
- Mackie E. J. Tenascin in connective tissue development and pathogenesis. Perspect Dev Neurobiol. 1994;2(1):125–132. [PubMed] [Google Scholar]
- Maguen E., Alba S. A., Burgeson R. E., Butkowski R. J., Michael A. F., Kenney M. C., Nesburn A. B., Ljubimov A. V. Alterations of corneal extracellular matrix after multiple refractive procedures: a clinical and immunohistochemical study. Cornea. 1997 Nov;16(6):675–682. [PubMed] [Google Scholar]
- Maseruka H., Ataullah S. M., Zardi L., Tullo A. B., Ridgway A. E., Bonshek R. E. Tenascin-cytotactin (TN-C) variants in pseudophakic/aphakic bullous keratopathy corneas. Eye (Lond) 1998;12(Pt 4):729–734. doi: 10.1038/eye.1998.178. [DOI] [PubMed] [Google Scholar]
- Maseruka H., Bonshek R. E., Tullo A. B. Tenascin-C expression in normal, inflamed, and scarred human corneas. Br J Ophthalmol. 1997 Aug;81(8):677–682. doi: 10.1136/bjo.81.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maseruka H., Ridgway A., Tullo A., Bonshek R. Developmental changes in patterns of expression of tenascin-C variants in the human cornea. Invest Ophthalmol Vis Sci. 2000 Dec;41(13):4101–4107. [PubMed] [Google Scholar]
- Mohan R. R., Liang Q., Kim W. J., Helena M. C., Baerveldt F., Wilson S. E. Apoptosis in the cornea: further characterization of Fas/Fas ligand system. Exp Eye Res. 1997 Oct;65(4):575–589. doi: 10.1006/exer.1997.0371. [DOI] [PubMed] [Google Scholar]
- Munier F. L., Korvatska E., Djemaï A., Le Paslier D., Zografos L., Pescia G., Schorderet D. F. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet. 1997 Mar;15(3):247–251. doi: 10.1038/ng0397-247. [DOI] [PubMed] [Google Scholar]
- Quantock A. J., Meek K. M. Proteoglycan distribution in the corneas of individuals with bullous keratopathy. Biochem Soc Trans. 1990 Oct;18(5):958–958. doi: 10.1042/bst0180958. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. T., Planck S. T., Huang X. N., Rich L., Ansel J. C. Detection of mRNA for the cytokines, interleukin-1 alpha and interleukin-8, in corneas from patients with pseudophakic bullous keratopathy. Invest Ophthalmol Vis Sci. 1995 Sep;36(10):2151–2155. [PubMed] [Google Scholar]
- Sage E. H., Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991 Aug 15;266(23):14831–14834. [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986 Dec;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Kawakatsu H., Ohta M., Saito M. Tenascin induction in tenascin nonproducing carcinoma cell lines in vivo and by TGF-beta 1 in vitro. J Cell Physiol. 1994 Jun;159(3):561–572. doi: 10.1002/jcp.1041590320. [DOI] [PubMed] [Google Scholar]
- Skonier J., Neubauer M., Madisen L., Bennett K., Plowman G. D., Purchio A. F. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol. 1992 Sep;11(7):511–522. doi: 10.1089/dna.1992.11.511. [DOI] [PubMed] [Google Scholar]
- Streeten B. W., Qi Y., Klintworth G. K., Eagle R. C., Jr, Strauss J. A., Bennett K. Immunolocalization of beta ig-h3 protein in 5q31-linked corneal dystrophies and normal corneas. Arch Ophthalmol. 1999 Jan;117(1):67–75. doi: 10.1001/archopht.117.1.67. [DOI] [PubMed] [Google Scholar]
- Takács L., Csutak A., Balázs E., Berta A. Immunohistochemical detection of betaIG-H3 in scarring human corneas. Graefes Arch Clin Exp Ophthalmol. 1999 Jul;237(7):529–534. doi: 10.1007/s004170050275. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Hammarback J. A., Jenrath D. A., Mackie E. J., Xu Y. Tenascin expression in the mouse: in situ localization and induction in vitro by bFGF. J Cell Sci. 1993 Jan;104(Pt 1):69–76. doi: 10.1242/jcs.104.1.69. [DOI] [PubMed] [Google Scholar]
- Tuori A., Uusitalo H., Thornell L. E., Yoshida T., Virtanen I. The expression of tenascin-X in developing and adult rat and human eye. Histochem J. 1999 Apr;31(4):245–252. doi: 10.1023/a:1003665712063. [DOI] [PubMed] [Google Scholar]
- Werth V. P., Williams K. J., Fisher E. A., Bashir M., Rosenbloom J., Shi X. UVB irradiation alters cellular responses to cytokines: role in extracellular matrix gene expression. J Invest Dermatol. 1997 Mar;108(3):290–294. doi: 10.1111/1523-1747.ep12286462. [DOI] [PubMed] [Google Scholar]
- Wheatley H. M., Traboulsi E. I., Flowers B. E., Maumenee I. H., Azar D., Pyeritz R. E., Whittum-Hudson J. A. Immunohistochemical localization of fibrillin in human ocular tissues. Relevance to the Marfan syndrome. Arch Ophthalmol. 1995 Jan;113(1):103–109. doi: 10.1001/archopht.1995.01100010105028. [DOI] [PubMed] [Google Scholar]


