Abstract
BACKGROUND/AIMS—The choroid, a low resistance vascular structure carrying 85% of the ocular blood flow, provides nourishment to and removal of potential toxic waste products from the adjacent non-vascularised outer layers of the retina, macula, and optic disc regions. Choroidal perfusion may be reduced in retinitis pigmentosa (RP) and might contribute to retinal pigment epithelium (RPE) degeneration. The aim of this study was to determine whether choroidal perfusion is reduced in RP and whether this is correlated with the stage of disease. METHODS—Ocular pulse amplitude (OPA) evaluated with the ocular blood flow (OBF) system, applanation intraocular pressure (IOP), visual fields, blood pressure (BP), and heart rate (HR) were measured in 75 RP patients having stage RP-I (stage I: visual field size: 7.85-14.67 cm2; n = 22), stage RP-II (stage II: visual field size: 2.83-7.84 cm2; n = 29), or stage RP-III (stage III: visual field size: 0.52-2.82 cm2; n = 24) were compared with matched healthy controls and each other. RESULTS—Neither IOP nor systemic perfusion parameters were significantly (p >0.1) altered, but OPA (mm Hg) in RP patients beginning with stage RP-II (1.6 (0.1), 27.3%, p<0.0001), and RP-III (1.2 (0.1), 45.5%, p<0.0001) was significantly reduced when compared with matched subgroups from a pool of healthy controls (2.2 (0.1), n = 94). CONCLUSIONS—OPA can be used neither for early clinical detection of RP nor to follow the natural course of the disease. However, our data show that in advanced stages of RP not only the retina but also the choroidal circulation is affected.
Full Text
The Full Text of this article is available as a PDF (129.1 KB).
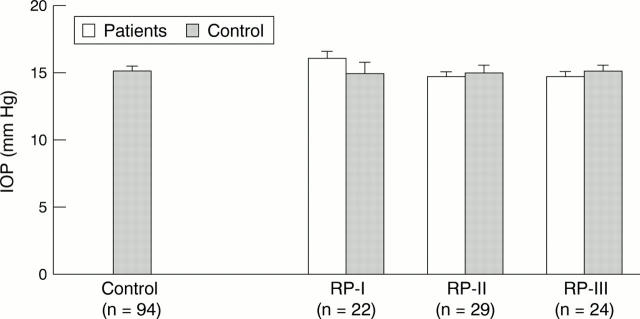
Figure 1 .
Intraocular pressures (IOP; mean (SEM)) of retinitis pigmentosa (RP) patients stages I (n = 22), II (n = 29), and III (n = 24), each compared with a matched control subgroup from a pool of healthy volunteers (n = 94).
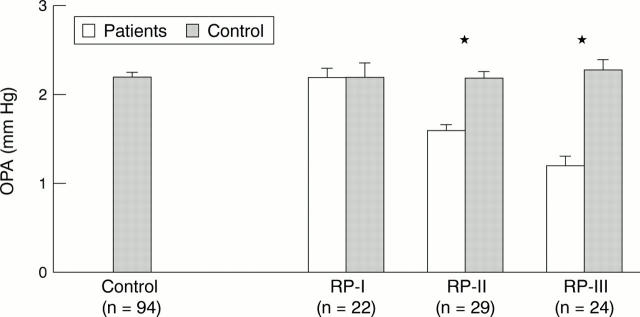
Figure 2 .
Ocular pulse amplitudes (OPA; mean (SEM)) of retinitis pigmentosa (RP) patients stages I (n = 22), II (n = 29), and III (n = 24), each compared with a matched control subgroup from a pool of healthy volunteers (n = 94). *Represents p <0.05 using Student's unpaired two tailed t test.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Nishimura J., Nakamura M., Kanaide H. Effects of nicorandil on cytosolic calcium concentrations and on tension development in the rabbit femoral artery. J Pharmacol Exp Ther. 1994 Feb;268(2):762–771. [PubMed] [Google Scholar]
- Abele A. E., Scholz K. P., Scholz W. K., Miller R. J. Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 1990 Mar;4(3):413–419. doi: 10.1016/0896-6273(90)90053-i. [DOI] [PubMed] [Google Scholar]
- Alm A., Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973 Jan 1;15(1):15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- Alm A., Bill A. The oxygen supply to the retina. II. Effects of high intraocular pressure and of increased arterial carbon dioxide tension on uveal and retinal blood flow in cats. A study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Acta Physiol Scand. 1972 Mar;84(3):306–319. doi: 10.1111/j.1748-1716.1972.tb05182.x. [DOI] [PubMed] [Google Scholar]
- Anderson T. J., Meredith I. T., Ganz P., Selwyn A. P., Yeung A. C. Nitric oxide and nitrovasodilators: similarities, differences and potential interactions. J Am Coll Cardiol. 1994 Aug;24(2):555–566. doi: 10.1016/0735-1097(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Berson E. L. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993 Apr;34(5):1659–1676. [PubMed] [Google Scholar]
- Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975 Jul;55(3):383–417. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- FRAYSER R., HICKAM J. B. EFFECT OF VASODILATOR DRUGS ON THE RETINAL BLOOD FLOW IN MAN. Arch Ophthalmol. 1965 May;73:640–642. doi: 10.1001/archopht.1965.00970030642008. [DOI] [PubMed] [Google Scholar]
- Flannery J. G., Farber D. B., Bird A. C., Bok D. Degenerative changes in a retina affected with autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1989 Feb;30(2):191–211. [PubMed] [Google Scholar]
- Frayser R., Saltzman H. A., Anderson B., Jr, Hickam J. B., Sieker H. O. The effect of hyperbaric oxygenation on retinal circulation. Arch Ophthalmol. 1967 Feb;77(2):265–269. doi: 10.1001/archopht.1967.00980020267023. [DOI] [PubMed] [Google Scholar]
- Gasser P., Flammer J. Short- and long-term effect of nifedipine on the visual field in patients with presumed vasospasm. J Int Med Res. 1990 Jul-Aug;18(4):334–339. doi: 10.1177/030006059001800411. [DOI] [PubMed] [Google Scholar]
- Goureau O., Hicks D., Courtois Y. Human retinal pigmented epithelial cells produce nitric oxide in response to cytokines. Biochem Biophys Res Commun. 1994 Jan 14;198(1):120–126. doi: 10.1006/bbrc.1994.1017. [DOI] [PubMed] [Google Scholar]
- Grunwald J. E., Maguire A. M., Dupont J. Retinal hemodynamics in retinitis pigmentosa. Am J Ophthalmol. 1996 Oct;122(4):502–508. doi: 10.1016/s0002-9394(14)72109-9. [DOI] [PubMed] [Google Scholar]
- Grøndahl J. Pericentral retinal dystrophy. Acta Ophthalmol (Copenh) 1987 Jun;65(3):344–351. doi: 10.1111/j.1755-3768.1987.tb08517.x. [DOI] [PubMed] [Google Scholar]
- Haefliger I. O., Flammer J., Lüscher T. F. Heterogeneity of endothelium-dependent regulation in ophthalmic and ciliary arteries. Invest Ophthalmol Vis Sci. 1993 Apr;34(5):1722–1730. [PubMed] [Google Scholar]
- Henkind P., Gartner S. The relationship between retinal pigment epithelium and the choriocapillaris. Trans Ophthalmol Soc U K. 1983;103(Pt 4):444–447. [PubMed] [Google Scholar]
- Hessemer V., Schmidt K. G. Influence of the vasodilator drug isosorbide dinitrate on ocular circulation. Arch Ophthalmol. 1997 Mar;115(3):324–327. doi: 10.1001/archopht.1997.01100150326003. [DOI] [PubMed] [Google Scholar]
- Langham M. E., Grebe R., Hopkins S., Marcus S., Sebag M. Choroidal blood flow in diabetic retinopathy. Exp Eye Res. 1991 Feb;52(2):167–173. doi: 10.1016/0014-4835(91)90256-e. [DOI] [PubMed] [Google Scholar]
- Langham M. E., Kramer T. Decreased choroidal blood flow associated with retinitis pigmentosa. Eye (Lond) 1990;4(Pt 2):374–381. doi: 10.1038/eye.1990.50. [DOI] [PubMed] [Google Scholar]
- Mehta J. L. Endothelium, coronary vasodilation, and organic nitrates. Am Heart J. 1995 Feb;129(2):382–391. doi: 10.1016/0002-8703(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Matsubara T., Takahashi K., Nakajima M., Nambu H., Tsubura A., Uyama M. [Angiographic findings and histological localization of indocyanine green in N-methyl-N-nitrosourea induced retinal degeneration in rats]. Nippon Ganka Gakkai Zasshi. 1999 Jul;103(7):489–496. [PubMed] [Google Scholar]
- Nyborg N. C., Prieto D., Benedito S., Nielsen P. J. Endothelin-1-induced contraction of bovine retinal small arteries is reversible and abolished by nitrendipine. Invest Ophthalmol Vis Sci. 1991 Jan;32(1):27–31. [PubMed] [Google Scholar]
- Osborne N. N., Barnett N. L., Herrera A. J. NADPH diaphorase localization and nitric oxide synthetase activity in the retina and anterior uvea of the rabbit eye. Brain Res. 1993 May 7;610(2):194–198. doi: 10.1016/0006-8993(93)91400-m. [DOI] [PubMed] [Google Scholar]
- Schmidt K. G., Mittag T. W., Pavlovic S., Hessemer V. Influence of physical exercise and nifedipine on ocular pulse amplitude. Graefes Arch Clin Exp Ophthalmol. 1996 Aug;234(8):527–532. doi: 10.1007/BF00184863. [DOI] [PubMed] [Google Scholar]
- Schmidt K. G., Rückmann A. V., Mittag T. W., Hessemer V., Pillunat L. E. Reduced ocular pulse amplitude in low tension glaucoma is independent of vasospasm. Eye (Lond) 1997;11(Pt 4):485–488. doi: 10.1038/eye.1997.131. [DOI] [PubMed] [Google Scholar]
- Schmidt K. G., von Rückmann A., Geyer O., Mittag T. W. Einfluss des Nifedipins auf die okuläre Pulsamplitude bei Normaldruckglaukom. Klin Monbl Augenheilkd. 1997 Jun;210(6):355–359. doi: 10.1055/s-2008-1035074. [DOI] [PubMed] [Google Scholar]
- Schmidt K. G., von Rückmann A., Mittag T. W. Okuläre Pulsamplitude bei okulärer Hypertension und verschiedenen Glaukomformen. Ophthalmologica. 1998;212(1):5–10. doi: 10.1159/000027250. [DOI] [PubMed] [Google Scholar]
- Schmidt K. G., von Rückmann A., Pillunat L. E. Topical carbonic anhydrase inhibition increases ocular pulse amplitude in high tension primary open angle glaucoma. Br J Ophthalmol. 1998 Jul;82(7):758–762. doi: 10.1136/bjo.82.7.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Louis P. J., Sulakhe P. V. Phosphorylation of cardiac sarcolemma by endogenous and exogenous protein kinases. Arch Biochem Biophys. 1979 Nov;198(1):227–240. doi: 10.1016/0003-9861(79)90414-4. [DOI] [PubMed] [Google Scholar]
- Tacke C. M., Pillunat L. E., Lang G. K. Okulare Kohlendioxid-reaktivität bei Retinopathia pigmentosa. Perimetrische Ergebnisse. Ophthalmologe. 1993 Oct;90(5):510–514. [PubMed] [Google Scholar]
- Tucker G. S., Jacobson S. G. Morphological findings in retinitis pigmentosa with early diffuse rod dysfunction. Retina. 1988;8(1):30–41. doi: 10.1097/00006982-198808010-00007. [DOI] [PubMed] [Google Scholar]
- Törnquist P., Alm A. Retinal and choroidal contribution to retinal metabolism in vivo. A study in pigs. Acta Physiol Scand. 1979 Jul;106(3):351–357. doi: 10.1111/j.1748-1716.1979.tb06409.x. [DOI] [PubMed] [Google Scholar]
- Ulrich C., Ulrich W. D., Vehlow K., Vehlow S. Hämodynamische Aspekte bei der Retinopathia pigmentosa (RP). Fortschr Ophthalmol. 1991;88(6):642–647. [PubMed] [Google Scholar]
- Wagner U., Wiederholt M. Membrane voltage and whole-cell currents in cultured pericytes of control rats and rats with retinal dystrophy. Curr Eye Res. 1996 Oct;15(10):1045–1053. doi: 10.3109/02713689609017654. [DOI] [PubMed] [Google Scholar]
- Williamson T. H., Harris A. Ocular blood flow measurement. Br J Ophthalmol. 1994 Dec;78(12):939–945. doi: 10.1136/bjo.78.12.939. [DOI] [PMC free article] [PubMed] [Google Scholar]