Abstract
The neuropeptide galanin colocalizes with choline acetyltransferase, the synthetic enzyme for acetylcholine, in a subset of cholinergic neurons in the basal forebrain of rodents. Chronic intracerebroventricular infusion of nerve growth factor induces a 3- to 4-fold increase in galanin gene expression in these neurons. Here we report the loss of a third of cholinergic neurons in the medial septum and vertical limb diagonal band of the basal forebrain of adult mice carrying a targeted loss-of-function mutation in the galanin gene. These deficits are associated with a 2-fold increase in the number of apoptotic cells in the forebrain at postnatal day seven. This loss is associated with marked age-dependent deficits in stimulated acetylcholine release, performance in the Morris water maze, and induction of long-term potentiation in the CA1 region of the hippocampus. These data provide unexpected evidence that galanin plays a trophic role to regulate the development and function of a subset of septohippocampal cholinergic neurons.
The 29 amino acid peptide galanin (1) colocalizes with choline acetyltransferase (ChAT) in 30–35% of cholinergic neurons in the medial septum and vertical limb diagonal band (VLDB) of the basal forebrain in the rat (2, 3). Most, if not all, of these galanin-positive cholinergic neurons project to the hippocampus (2, 4). These findings have led to a number of functional studies addressing the role played by galanin in the basal forebrain cholinergic system, including its effects on acetylcholine (ACh) release as well as learning and memory. Acute administration of galanin into the hippocampus or third ventricle of rodents inhibits scopolamine-induced ACh release in a dose-dependent manner and is reversed by the coadministration of the chimeric-peptide galanin receptor antagonists M15 and M40 (5, 6). Centrally administered galanin also has inhibitory effects on several tests of learning and memory (7, 8). In contrast to these inhibitory actions, exogenous galanin has no effect on the increased release of ACh that occurs when a rodent is exposed to a novel environment (9). Neither of the galanin antagonists has an effect on ACh release or on cognition in the absence of exogenously administered galanin (5, 6). Similarly, whereas exogenous galanin inhibits long-term potentiation (LTP) in hippocampal CA1 slices that is reversed by the M40 galanin antagonist, M40 has no effect on LTP when applied alone (10). In addition, there is increasing evidence that the M15 and M40 ligands may act as partial agonists in the hippocampus (11, 12) and as full agonists in vitro to the cloned galanin receptor subtypes (13). These somewhat conflicting data emphasize the limitations of the pharmacological tools that are currently available and cast some doubt on the role played by endogenously secreted galanin in the modulation of steady-state ACh release.
We have recently generated mice carrying a loss-of-function mutation in the galanin gene (14) and have demonstrated that galanin is (i) essential for the developmental survival of a subset of cells in the dorsal root ganglia, and (ii) a neuritogenic factor to regenerating sensory neurons (15). We have now used these galanin knockout animals to further define the role played by galanin within the cholinergic basal forebrain system and its effects on memory and cognition. In this paper, we demonstrate that the chronic absence of galanin throughout development results in the loss of a third of the cholinergic septohippocampal neurons. Cell loss by apoptosis appears to occur at P7. These deficits are associated with marked age-dependent deficits in stimulated ACh release, performance in the Morris water maze, and induction of LTP in the CA1 region of the hippocampus. These data provide unexpected evidence that galanin, like nerve growth factor (NGF), plays a trophic role to a subset of septohippocampal cholinergic neurons. Because NGF is known to induce galanin gene expression in the forebrain, it is possible that the two proteins function together in a molecular cascade to regulate basal forebrain cholinergic survival and function.
Methods
Mutant Animals.
All experiments were performed on 4- and 10-month-old male mice homozygous for a targeted mutation in the galanin gene. Age-matched wild-type littermates were used as controls in all experiments. Details of the exact strain and breeding history of the colony have previously been published (14, 16). In brief, the generation of the galanin knockout mice was performed by using the E14 cell line; the colony has remained inbred on the 129olaHsd strain and is currently at F16. All animals were fed standard chow and water ad libitum. Animal care and procedures were carried out within United Kingdom Home Office protocols and guidelines. In all cases, experiments and data analysis were performed with the observer blinded to the genotype of the animals studied. All data are presented as mean ± SEM.
Stereological and Histological Analysis.
For stereological cell counting, animals were perfused transcardially with 4% paraformaldehyde in phosphate buffer (pH 7.4), cryoprotected in 30% sucrose in phosphate buffer at 4°C, and cut frozen at 40-μm thickness on a sliding knife microtome. Immunohistochemistry was performed by using a human polyclonal ChAT (1:1,000; a gift of L. Hirsch, University of Kentucky) and a polyclonal TrkA (1:10,000; a gift of L. Reichardt, Howard Hughes Foundation, University of California, San Francisco) antibodies and visualized by metallic intensification of diaminobenzidine, as previously reported (17, 18). The total number of ChAT and TrkA immunopositive neurons within the cholinergic basal forebrain subfields was determined by using the optical fractionator, as previously reported (18). Briefly, the basal forebrain was outlined at low magnification (×4 objective), and at least 30% of the outlined region was measured with a systematic random design by using dissector frames (442 μm) with a ×100 planar oil immersion lens with a 1.4 n.a. The average section thickness was 30 μm. However, neurons were counted only within a 20-μm tissue height with guard heights of 5 μm at the top and bottom of each section. As previously demonstrated, antibody penetration was uniform throughout the entire thickness of the section (18).
Cellular apoptosis was measured by using age-matched mutant and wild-type mice. The brains were removed and fixed by immersion in 4% paraformaldehyde overnight at 4°C. Tissue was cryo preserved in 20% sucrose, sectioned at 30 mm on a cryostat, and processed with terminal deoxynucleotidyltransferase-mediated dUTP–biotin nick-end labeling (TUNEL) to label apoptotic cells (Boehringer Mannheim). Sections were counterstained by using hematoxylin and were mounted. The total number of TUNEL-positive cells was manually counted in the five adjacent sections that covered the entire medial septum and VLDB.
ChAT Activity.
Three hundred-micrometer sections of mouse brain were cut on a cryostat and were kept frozen while the medial septum and diagonal band were punched out and pooled. Two hundred microliters of 10 mM phosphate buffer, pH 7.4, containing 0.1% Triton X-100 was added and the tissue homogenized on ice in a 1.5-ml Eppendorf by 20 strokes of a close-fitting pestle. Two microliters of sample was used in the assay. ChAT activity was measured as previously described (19). Two microliters of homogenate was pipetted into Durham tubes on ice in triplicate. Blanks, in duplicate, were boiled for 15 min. Five microliters of substrate mix was added [final concentrations: choline bromide (10 mM)/sodium chloride (300 mM)/sodium phosphate buffer, pH 7.4, (50 mM)/EDTA (10 mM)/eserine sulfate (0.1 mM)/14C acetyl CoA (0.1 mM)] sequentially to tubes and incubated at 37°C. After 10 min, tubes were washed out with 1 ml ice-cold phosphate buffer. Four hundred microliters of Kalignost (acetonitrile with 0.5% tetraphenylboron) and subsequently scintillant were added. 14C-ACh produced extracted into the organic phase and radioactivity was measured by using a β-scintillation counter. Protein estimations were carried out by using the Bio-Rad Protein Assay. Activity is expressed as femtomol of ACh produced/minute/milligram protein and is corrected for genotype-related changes in the number of ChAT-IR-positive neurons (see Table 1).
Table 1.
Mean total number of ChAT- and TrkA-immunoreactive neurons within basal forebrain subfields in wild-type and galanin mutant mice 4 months of age
| Genotype | Marker | Medial septum | Vertical limb DB | Horizontal limb DB | Nucleus Basalis |
|---|---|---|---|---|---|
| +/+ | ChAT | 2,645 ± 224 | 1,966 ± 295 | 1,735 ± 244 | 10,357 ± 586 |
| TrkA | 1,990 ± 147 | 1,785 ± 152 | 2,181 ± 233 | 6,599 ± 288 | |
| −/− | ChAT | 1,740 ± 272 | 1,262 ± 196 | 1,305 ± 76 | 9,184 ± 828 |
| TrkA | 1,223 ± 168 | 1,235 ± 145 | 1,966 ± 289 | 5,412 ± 291 | |
| % reduction | ChAT | 34%** | 36%** | 25% | 11% |
| +/+ vs −/− | TrkA | 39%** | 31%* | 10% | 20%* |
Statistically significant differences are noted for both markers in the medial septum and VLDB (t test, *, P < 0.05, **, P < 0.01, n = 6).
ACh Release Measured by in Vivo Microdialysis in the Hippocampus.
Microdialysis experiments were performed on awake freely moving mice. One day before the experiment, animals were anesthetized with pentobarbital (60 mg/kg i.p.) and fixed in a steretotaxic frame. A CMA/7 microdialysis probe, 2-mm membrane length, was implanted into the right hippocampus at coordinates: AP D −3.0 mm, L + 3.0 mm, V −3.8 mm from the brain surface (20). A small microscrew was placed into the skull and the whole assembly secured with the dental cement (Dentalon Plus, Heraeus, Germany). The following day, the probe was perfused with Ringer solution (147 mM NaCl/2.3 mM CaCl2/4 mM KCl) containing 10 μM neostigmine at a flow rate of 1.25 μl/min. The first 100- to 120-min dialysates were discarded, and then fractions were collected at 20-min intervals. ACh release was stimulated by perfusions with Ringer containing 1 μM scopolamine⋅HBr. ACh in the microdialysis samples was determined by microbore liquid chromatography/electrochemistry on peroxidase redox polymer coated electrodes, as previously described (21).
Behavioral Testing.
A water maze was constructed with white fiberglass to form a circular pool (1 M in diameter) and was filled with water (24 ± 1°C) rendered opaque by a white food dye. A submerged platform (9 cm diameter) was positioned in the northeast quadrant and was submerged 1 cm below the surface of the water. A closed-circuit video camera, fitted with a wide-angle lens, was mounted above the center of the pool. This was connected to an image analyzer that digitized the image and relayed it to a personal computer running the watermaze software package supplied by HVS Ltd., Cambridge, U.K.. Each swim trial began when the mouse was placed in the water maze at one of the four poles and ended when it climbed onto the hidden platform. The time taken to locate the submerged platform on each trial was recorded. If the mouse had not climbed onto the platform within 60 s of the beginning of the trial, the experimenter placed it there. The mouse remained on the platform for 30 s, after which it was removed to a high-sided opaque plastic container for a further 30 s. Each mouse was trained with two trials per day for 20 days, and the mean latencies to escape to the hidden platform (placed in the northeast quadrant of the pool) were analyzed in 2-day blocks. The point of entry was randomly assigned on each trial. At the end of the acquisition phase, the spatial memory of each mouse was assessed in a probe trial in which the submerged platform was removed from the pool, and the animal was allowed to swim freely in the pool for 60 s.
Electrophysiology.
Mouse brains were dissected and placed in ice-cold artificial cerebrospinal fluid (124 mM NaCl/3 mM KCl/1 mM MgSO4/1.25 mM NaH2PO4/26 mM NaH2CO3/2 mM CaCl2/10 mM d-glucose) that was saturated with 95% CO2:5% O2⋅350-μm-thick transverse hippocampal slices were prepared by using a Campden vibroslicer and two to three of these immediately transferred to a submersion-type chamber at 32°C. Extracellular field responses were recorded by using thin glass electrodes (0–5 MΩ) filled with 4 M NaCl and placed in stratum radiatum or oriens in the CA1 area of the hippocampus. Intracellular sharp electrode recordings were made in current clamp by using 60–100 MΩ electrodes filled with 2 M potassium methylsulphate. Reproducible postsynaptic excitatory and/or inhibitory synaptic responses were evoked by repetitive afferent stimulation (0.67 Hz) of the same dendritic field by using bipolar nickel-chromium electrodes. Porcine galanin used at 100 nM was obtained from Peninsula Laboratories.
Results
A Subpopulation of Cholinergic Neurons Are Lost by Increased Cell Death in the Basal Forebrain of Galanin Mutant Mice.
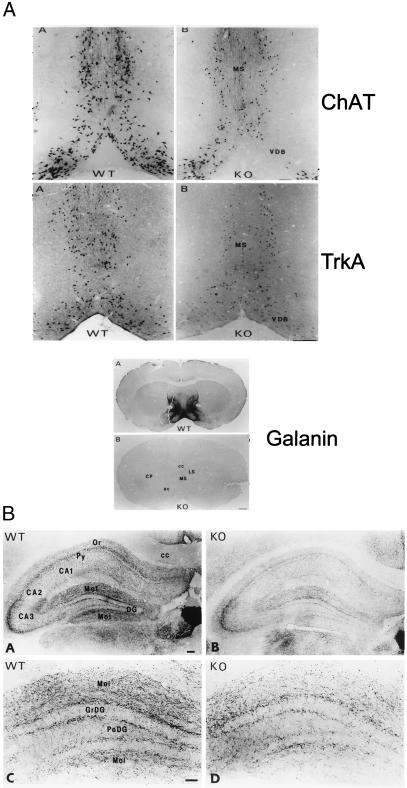
To determine whether the absence of galanin throughout pre- and postnatal brain development affects the total number of cholinergic neurons in the basal forebrain, stereological counting of ChAT and high-affinity nerve growth factor receptor (TrkA) immunopositive cells, both of which are markers of cholinergic neurons, was performed on adult (4-month-old) animals. Approximately one-third of the cholinergic neurons in the medial septum and VLDB are absent (Fig. 1A). Quantitation of these deficits (Table 1) demonstrates that the loss of both cholinergic markers is highly significant (P < 0.01) and is specific to the septohippocampal neurons. Consistent with this, a marked decrease in ChAT staining was noted in the septohippocampal cholinergic projections to the CA1 and CA2 fields and the molecular layers of the hippocampus (Fig. 1B).
Figure 1.
(A) Representative photomicrographs of adjacent basal forebrain sections in wild-type and mutant animals stained for ChAT and TrkA, respectively, demonstrating that one-third of cholinergic neurons are absent in the medial septum and VLDB of mutants. (B) Photomicrographs of low- and high-power fields of the septal/diagonal band cholinergic projections to the hippocampus of wild-type and mutant animals stained for ChAT. Results demonstrate a striking reduction in cholinergic innervation within the CA1 and CA2 fields and the molecular layers of the hippocampus in the mutant animals. CA1, CA2, and CA3, hippocampal subfields; cc, corpus callosum; DG, dentate gyrus; GrDG, granular layer of the DG; Mol, molecular layer of the hippocampus; Or, stratum oriens; PoDG, polymorphic layer of the DG; Py, pyramidal cell layer of the hippocampus.
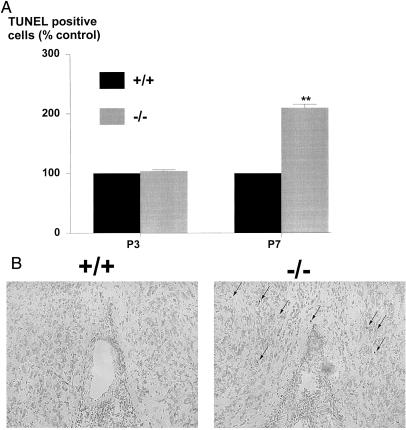
We hypothesized that the reduced populations of cholinergic neurons in the basal forebrain of the mutant mice might be caused by an increased loss of cells by apoptosis. To address this and to define the time point during development when the cell loss in the medial septum and VLDB occurred, we visualized apoptosis in these subfields of age-matched wild-type and mutant neonates by using the TUNEL method. No differences were noted in the number of TUNEL-positive cells at postnatal day three (P3) in age-matched wild-type and mutant animals (Fig. 2A). In contrast, a highly significant 2.1-fold increase in the number of TUNEL-positive cells was noted at P7 (Fig. 2 A and B) in the mutants, suggesting that there is a concerted wave of cell death that occurs because of the absence of galanin.
Figure 2.
(A) The number of TUNEL-positive cells in medial septum and VLDB sections from mutant and wild-type animals at various time points during postnatal development was manually counted. The results are expressed as a percentage of age-matched wild-type controls demonstrating a wave of apoptosis in the mutants at P7 that was not seen at P3 (t test, **P < 0.01, n = 6). (B) Representative photomicrographs of basal forebrain sections in wild-type and mutant animals, demonstrating a number of TUNEL-positive cells (arrows) in the mutant section that is not present in the wild-type control.
Galanin Mutant Mice Demonstrate Age-Dependent Deficits in ACh Release and Cognition.
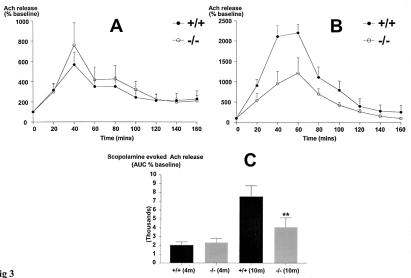
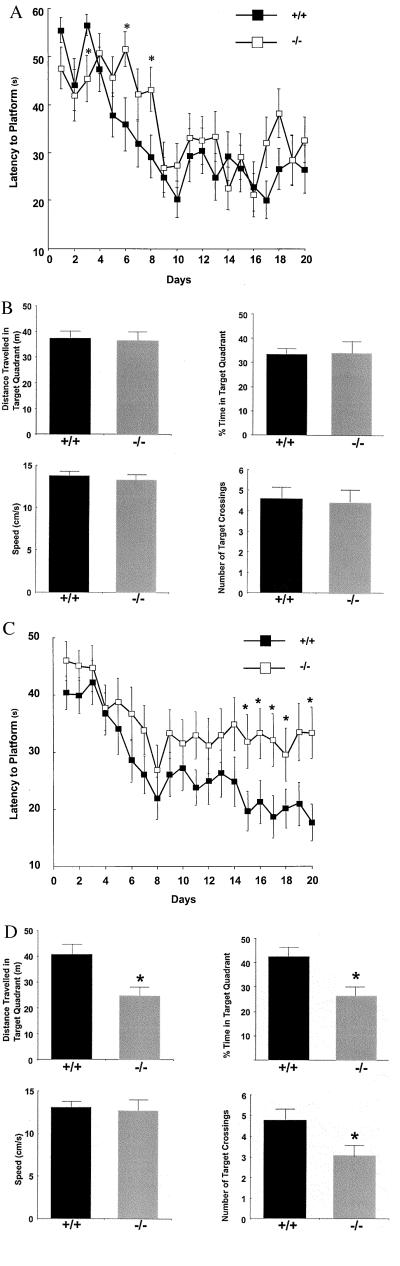
Basal forebrain ChAT activity and hippocampal ACh release in conscious freely moving 4-month-old animals were measured, because immunohistochemical detection of steady-state protein levels of forebrain and hippocampal ChAT do not equate to activity of the synthetic enzyme or the release of its product. These data reveal a 66% increase in ChAT activity in the mutants (22 ± 0.7 vs. 13 ± 0.6 fmol/min/mg protein/cholinergic neuron, mutant and wild-type, respectively; t test P < 0.01, n = 8), associated with an unchanged response to scopolamine stimulation of ACh release (Fig. 3A). These results imply that the remaining cholinergic cells have up-regulated ChAT activity to compensate for the developmental loss of a subset of cholinergic neurons, increasing ACh release. As might be expected, no differences were noted in the performance in the Morris water maze in young adult mutant animals compared with age-matched wild-type controls (Fig. 4 A and B).
Figure 3.
(A) The effect of 40-min perfusion of 1 μM scopolamine on ACh release in the hippocampus of wild-type and mutant animals at 4 months of age was studied. No significant differences in stimulated extracellular ACh levels were noted in the wild-type and mutant groups (n = 6). In both groups, the released ACh slowly returned to the basal values within 80–100 min. (B) In the 10-month-old animals, scopolamine-evoked ACh release was significantly attenuated in the mutants compared with wild-type age-matched controls (t test, **P < 0.01; n = 5). (C) Similarly, the area under the curve (AUC 0–120 min) values was not significantly different between the genotypes at 4 months of age but was reduced by 47% in the 10-month-old animals (t test, **P < 0.01; n = 5).
Figure 4.
(A) The performance of 4-month-old mutant mice (n = 20) in the water maze is unchanged to that of 10-month-old matched wild-type controls. An ANOVA on the mean latencies to escape with factors of genotype and blocks revealed no significant main effect of genotype at 4 months of age. (B) During the probe trial in which the platform was removed and the mice swam freely for 60 s, there were no significant differences between the 4-month-old galanin mutant mice and the age-matched wild-type control mice in the speed at which they swam in the maze, the time they spent in the northeast quadrant, the number of times they crossed the exact position of the platform, and the distance they swam in the northeast quadrant. At this age, both groups spent over 33% of their time during the probe trial in the northeast quadrant, indicating both had encoded the position of the platform relative to the spatial cues provided. (C) In contrast, at 10 months of age, the performance of the wild-type control mice (n = 20) was significantly better from day 15 onward (P < 0.05) than that of the galanin mutant mice. (D) The behavior of the 10-month-old galanin mutant mice was also significantly worse in the probe trial than that of the wild-type controls, in that they spent significantly less time in the northeast quadrant (P < 0.05, F = 9.04), swam a shorter distance in the northeast quadrant (P < 0.05, F = 9.30), and made significantly fewer crossings of the exact location of the platform at this age (P < 0.05, F = 4.98). These differences could not be accounted for by the speed at which they swam, as this did not significantly differ from that of the wild-type controls, suggesting an impairment in learning or memory.
Several studies have demonstrated an age-dependent reduction in the number of forebrain cholinergic neurons and ChAT activity in the rat (22, 23). We therefore tested whether the combination of developmental and age-related losses might reveal deficits in stimulated ACh release and the Morris water maze in older mutant animals. A 50% decrease in scopolamine-stimulated ACh release was observed in 10-month-old mutants (Fig. 3 B and C), which was paralleled by significant deficits in performance in the Morris water maze in mutant animals when compared with age-matched wild-type controls (Fig. 4 C and D). Of note, the differences in mean latency to the platform and time in the target quadrant during the probe test were not explained by differences in swim speed between the two genotypes (Fig. 4D) nor by changes in motor function, as performance in the rotarod test was unaffected by genotype (data not shown).
LTP Deficits in the Galanin Mutant Mice Are Specific to the Stratum Oriens.
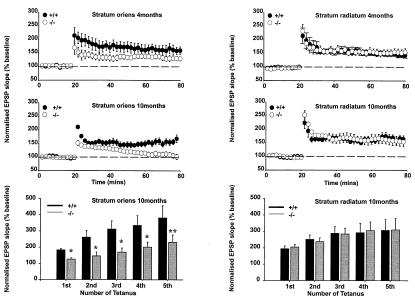
As the hippocampus is strongly implicated in spatial learning tasks such as the water maze, we next investigated whether the disruption in cognitive performance was reflected in dysfunctions in the electrophysiological properties of the CA1 region of mutant animals (24). According to all of the criteria tested, the synaptic and membrane properties of hippocampal neurons of 4-month-old mutant mice were apparently normal. Thus, equivalent glutamatergic and GABAergic synaptic responses were evoked by similar intensities of stimulation in the Schaffer collateral commissural pathway in both stratum oriens and stratum radiatum. Neurons exhibited similar resting membrane potentials, input resistances, and spike frequency adaptation in the two genotypes (data not shown). Similarly, no differences in 4-month-old mutants were noted in paired-pulse depression of population spikes or paired-pulse facilitation of field excitatory postsynaptic potentials (EPSPs) in either pathway or the slow muscarinic receptor-mediated EPSP (EPSPM), which are most marked in the stratum oriens (25). In contrast, although tetanically (100 Hz for 1 s) induced LTP in the stratum radiatum (apical dendrites) appeared normal (Fig. 5A Right), the magnitude of LTP in the stratum oriens (basal dendrites) was reduced in mutant mice when compared with wild-type controls (Fig. 5A Left). This deficit was more pronounced in 10-month-old animals, such that in this group, prolonged short-term potentiation and not LTP was induced by 100 Hz for 1 s tetanus (Fig. 5B Left). Analysis of the profile of LTP saturation induced by repeated tetanic stimulation in the 10-month-old animals also revealed marked deficits in the oriens that were not present in stratum radiatum (Fig. 5C).
Figure 5.
Comparative LTP profiles in the stratum oriens (n = 6, left-hand graphs) and stratum radiatum (n = 9, right-hand graphs) in 4-month- (A Top) and 10-month-old (B Middle) wild-type and mutant animals induced by 100 Hz for 1 s tetanus. LTP is selectively impaired in the stratum oriens, and the magnitude of the deficit is increased with age. (C) The bar graphs (Bottom) illustrate LTP saturation profiles in the stratum oriens and radiatum of the hippocampal CA1 region in a separate group of 10-month-old mutant and wild-type mice (n = 4). These profiles were generated by delivering a series of tetani, each comprising 100 Hz for 1 s, each tetanus separated from the next by a 30-min interval. No significant differences were noted in the LTP saturation profiles in the stratum radiatum (P > 0.05). In contrast, the magnitude of LTP induced by each consecutive tetanus in the stratum oriens of mutant animals was smaller than that observed in wild-type mice (t test, *, P < 0.05, **, P < 0.01).
Discussion
The cholinergic basal forebrain is critically involved in cognition and undergoes marked degeneration in Alzheimer's disease (26). NGF is the only factor known to be crucial to both the development and maintenance of the cholinergic basal forebrain system and is retrogradely transported from the hippocampus to the forebrain (27). Chronic intracerebroventricular infusion of NGF up-regulates ChAT gene expression and activity in the forebrain, thereby increasing ACh release (28–30). The trophic role played by NGF is evident because it prevents the death of cholinergic neurons in vivo after axotomy (31, 32) and reverses the cognitive deficits associated with lesion-induced damage to cholinergic pathways (33). Further, targeted disruption of the high-affinity NGF receptor TrkA results in a developmental loss of 40% of ChAT-IR neurons in the medial septum associated with a doubling of the number of TUNEL-positive neurons in the basal forebrain at P7 (34). Loss of one NGF allele also results in cholinergic deficits associated with a decreased performance in the Morris maze (35).
In rats, one-third of the cholinergic septohippocampal neurons contain neuropeptide galanin, and chronic intracerebroventricular infusion of NGF specifically induces galanin gene expression 3- to 4-fold in these neurons (36). Our present findings demonstrate that the pre- and postnatal absence of galanin causes deficits in cholinergic cell number in the basal forebrain of a similar magnitude and distribution to that noted in mice that lack TrkA. Further, losses in both transgenic strains of mice appear to be mediated by apoptotic mechanisms and to occur at the same postnatal time point. These data are therefore consistent with the hypothesis that NGF and galanin function together in a molecular cascade to regulate basal forebrain cholinergic survival and function. Further, the finding that the neuropeptide galanin plays a developmental role to the basal forebrain is not specific to the central nervous system. Galanin is also essential for the postnatal developmental survival of a subset of sensory neurons in the dorsal root ganglion (15). The cell loss occurs at P3/4 and is associated with a marked diminution in neuropathic pain behavior in adult mutants (16).
The actions of galanin as a trophic factor to basal forebrain cholinergic neurons are likely to be mediated by the activation of galanin receptors. One would therefore expect that the neurons, which depend on galanin for their survival, would express one or more of the galanin receptor subtypes at some point in their development. Miller et al. failed to demonstrate colocalization of the mRNA for the first galanin receptor subtype (GALR1) with ChAT in the basal forebrain cholinergic neurons (37). Most recently, data from O'Donnell et al. demonstrate that the newly cloned galanin receptor subtype, designated GALR2, is also expressed at the mRNA level in the medial septum and VLDB (38). The same study demonstrated high levels of GALR1 mRNA in the CA1 fields of the hippocampus but failed to detect GALR2 in this region (38). Furthermore, recent data that the two receptor subtypes couple to completely different signal transduction pathways (39–42) imply that the receptors may function in divergent molecular cascades and may play separate roles relating to cellular growth and survival. At present, antisera to the cloned receptor subtypes are unavailable, thus expression studies at the protein level are not yet possible.
Irrespective of the exact mechanism underlying the developmental loss of one-third of the cholinergic septohippocampal neurons in the galanin mutant animals, the resulting deficits may provide an explanation for the observed phenotypic abnormalities in the water maze. Previous data using the selective cholinergic neurotoxin192-IgG-saporin indicated that cholinergic deficits had little effect on tests of reference memory such as the Morris water maze (43, 44). It should be noted, however, that in these studies destruction of the medial septum and VLDB cholinergic neurons was by no means complete. Moreover, the majority of these studies were performed on adult animals, as compared with the more plastic developing neonatal basal forebrain cholinergic system. The data reported above might therefore be interpreted as indicating that selective and repeated lesions by using the toxin at varying developmental phases might have profound effects on hippocampal-dependent processes. Compatible with this, neonatal lesioning with the toxin administered shortly after birth (P4), which is then repeated in adulthood, causes deficits in the water maze (45). It should also be noted that our present data in no way exclude the possibility that the early loss of ChAT containing septal/diagonal band neurons may well precipitate a reorganization/plasticity response within this region. Further, we cannot exclude similar developmental deficits in the hippocampus or in other brain regions of mutant animals that may also contribute to the observed cognitive deficits.
As with the water maze results, hippocampal deficits and/or reorganization may also contribute to impairment in LTP. The specificity of the LTP deficits to the stratum oriens may relate to the observations of a number of groups that the CA1 basal dendrites receive a greater cholinergic input from the medial septum and express higher levels of galanin than the apical dendritic arbor (2, 46). Therefore, the developmental loss of galanin-responsive cells projecting to the oriens would have a greater effect on this dendritic arbor than the radiatum. Lastly, it is also possible that the loss of the direct inhibitory influence of galanin on LTP, or an increased associative influence of cholinergic inputs on LTP, in the adult may provide a further explanation for our observations that LTP saturates more quickly in the mutant animals (Fig. 5B).
The relative importance of the role played by galanin during development compared with neuromodulatory effects in the adult is central to the apparent paradox between the existing pharmacological data and the present findings using the galanin mutant animals. Most published pharmacological data indicate that endogenous galanin release has little or no effect on cognition or LTP in the intact rodent. It is therefore possible that, in the adult, the predominant role played by galanin is during states of high rates of neuronal firing such as injury or anoxic damage. In these situations, galanin would limit injury by acting both as a trophic factor and as an inhibitory neuromodulator, reducing the release of the excitatory amino acids glutamate and aspartate (47). Compatible with this hypothesis, endogenous galanin release from the hippocampus is markedly increased by electrical stimulation of the basal forebrain (48).
Acknowledgments
We thank Professor J Crawley (National Institute of Mental Health) for helpful discussions and advice at many stages of these studies and J. Syed for histological assistance. This work was supported by grants from the Medical Research Council and National Institutes of Health (AG10688 and AG09466).
Abbreviations
- ChAT
choline acetyltransferase
- VLDB
vertical limb diagonal band
- ACh
acetylcholine
- LTP
long-term potentiation
- NGF
nerve growth factor
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP–biotin nick-end labeling
- Pn
postnatal day n
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210254597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210254597
References
- 1.Tatemoto K, Rokaeus A, Jornvall H, McDonald T J, Mutt V. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 2.Melander T, Staines W A, Rokaeus A. Neuroscience. 1986;19:223–240. doi: 10.1016/0306-4522(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 3.Melander T, Staines W A, Hokfelt T, Rokaeus A, Eckenstein F, Salvaterra P M, Wainer B H. Brain Res. 1985;360:130–138. doi: 10.1016/0006-8993(85)91228-4. [DOI] [PubMed] [Google Scholar]
- 4.Gaykema R P, van-der-Kuil J, Hersh L B, Luiten P G. Neuroscience. 1991;43:349–360. doi: 10.1016/0306-4522(91)90299-4. [DOI] [PubMed] [Google Scholar]
- 5.Bartfai T, Langel U, Bedecs K, Andell S, Land T, Gregersen S, Ahren B, Girotti P, Consolo S, Corwin R, et al. Proc Natl Acad Sci USA. 1993;90:11287–11291. doi: 10.1073/pnas.90.23.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartfai T, Bedecs K, Land T, Langel U, Bertorelli R, Girotti P, Consolo S, Xu X J, Wiesenfeld H Z, Nilsson S, et al. Proc Natl Acad Sci USA. 1991;88:10961–10965. doi: 10.1073/pnas.88.23.10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald M P, Wenk G L, Crawley J N. Behav Neurosci. 1997;111:552–563. doi: 10.1037//0735-7044.111.3.552. [DOI] [PubMed] [Google Scholar]
- 8.Ogren S O, Kehr J, Schott P A. Neuroscience. 1996;75:1127–1140. doi: 10.1016/0306-4522(96)00215-1. [DOI] [PubMed] [Google Scholar]
- 9.Taber M T, Crawley J N. Psychobiology. 1999;27:57–62. [Google Scholar]
- 10.Sakurai E, Maeda T, Kaneko S, Akaike A, Satoh M. Neurosci Lett. 1996;212:21–24. doi: 10.1016/0304-3940(96)12772-5. [DOI] [PubMed] [Google Scholar]
- 11.Antoniou K, Kehr J, Snitt K, Ogren S O. Br J Pharmacol. 1997;121:1180–1186. doi: 10.1038/sj.bjp.0701233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H Y, Wild K D, Shank R P, Lee D H S. Neuropeptides. 1999;33:197–205. doi: 10.1054/npep.1999.0024. [DOI] [PubMed] [Google Scholar]
- 13.Smith K E, Forray C, Walker M W, Jones K A, Tamm J A, Bard J, Branchek T A, Linemeyer D L, Gerald C. J Biol Chem. 1997;272:24612–24616. doi: 10.1074/jbc.272.39.24612. [DOI] [PubMed] [Google Scholar]
- 14.Wynick D, Small C J, Bacon A, Holmes F E, Norman M, Ormandy C J, Kilic E, Kerr N C H, Ghatei M, Talamantes F, et al. Proc Natl Acad Sci USA. 1998;95:12671–12676. doi: 10.1073/pnas.95.21.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes F E, Mahoney S, King V R, Bacon A, Pachnis V, Curtis R, Priestley J V, Wynick D. Proc. Natl. Acad. Sci. USA. 2000. , 11563–11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr B J, Cafferty W B J, Gupta Y K, Bacon A, Wynick D, McMahon S B, Thompson S W N. Eur J Neurosci. 2000;12:793–802. doi: 10.1046/j.1460-9568.2000.00967.x. [DOI] [PubMed] [Google Scholar]
- 17.Sobreviela T, Clary D O, Reichardt L F, Brandabur M M, Kordower J H, Mufson E J. J Comp Neurol. 1994;350:587–611. doi: 10.1002/cne.903500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilmore M L, Erickson J D, Varoqui H, Hersh L B, Bennett D A, Cochran E J, Mufson E J, Levey A I. J. Comp. Neurol. 1999. 693–704. [PubMed] [Google Scholar]
- 19.Allen S J, MacGowan S H, Tyler S, Wilcock G K, Robertson A G, Holden P H, Smith S K, Dawbarn D. Neurosci Lett. 1997;239:33–36. doi: 10.1016/s0304-3940(97)00872-0. [DOI] [PubMed] [Google Scholar]
- 20.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 21.Kehr J, Dechent P, Kato T, Ogren S O. J Neurosci Methods. 1998;83:143–150. doi: 10.1016/s0165-0270(98)00074-0. [DOI] [PubMed] [Google Scholar]
- 22.Luine V N, Renner K J, Heady S, Jones K J. Neurobiol Aging. 1986;7:193–198. doi: 10.1016/0197-4580(86)90042-4. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong D M, Sheffield R, Buzsaki G, Chen K S, Hersh L B, Nearing B, Gage F H. Neurobiol Aging. 1993;14:457–470. doi: 10.1016/0197-4580(93)90104-j. [DOI] [PubMed] [Google Scholar]
- 24.Davies C H, Collingridge G L. J Physiol (London) 1996;496:451–470. doi: 10.1113/jphysiol.1996.sp021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole A E, Nicoll R A. J Physiol (London) 1984;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitehouse P J, Price D L, Struble R G, Clark A W, Coyle J T, DeLong M R. Science. 1982;215:1237–1239. [Google Scholar]
- 27.Sofroniew M V, Galletly N P, Isacson O, Svendsen C N. Science. 1990;247:338–342. doi: 10.1126/science.1688664. [DOI] [PubMed] [Google Scholar]
- 28.Higgins G A, Koh S, Chen K S, Gage F H. Neuron. 1989;3:247–256. doi: 10.1016/0896-6273(89)90038-x. [DOI] [PubMed] [Google Scholar]
- 29.Cavicchioli L, Flanigan T P, Dickson J G, Vantini G, Dal Toso R, Fusco M, Walsh F S, Leon A. Brain Res Mol Brain Res. 1991;9:319–325. doi: 10.1016/0169-328x(91)90079-d. [DOI] [PubMed] [Google Scholar]
- 30.Rylett R J, Goddard S, Schmidt B M, Williams L R. J Neurosci. 1993;13:3956–3963. doi: 10.1523/JNEUROSCI.13-09-03956.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montero C N, Hefti F. Neurobiol Aging. 1989;10:739–743. doi: 10.1016/0197-4580(89)90011-0. [DOI] [PubMed] [Google Scholar]
- 32.Montero C N, Hefti F. J Neurosci. 1988;8:2986–2999. doi: 10.1523/JNEUROSCI.08-08-02986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer W, Wictorin K, Bjorklund A, Williams L R, Varon S, Gage F H. Nature (London) 1987;329:65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- 34.Fagan A M, Garber M, Barbacid M, Silos-Santiago I, Holtzman D M. J Neurosci. 1997;17:7644–7654. doi: 10.1523/JNEUROSCI.17-20-07644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen K S, Nishimura M C, Armanini M P, Crowley C, Spencer S D, Phillips H S. J Neurosci. 1997;17:7288–7296. doi: 10.1523/JNEUROSCI.17-19-07288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planas B, Kolb P E, Raskind M A, Miller M A. J Comp Neurol. 1997;379:563–570. [PubMed] [Google Scholar]
- 37.Miller M A, Kolb P E, Planas B, Raskind M A. J Comp Neurol. 1998;391:248–258. [PubMed] [Google Scholar]
- 38.O'Donnell D, Ahmad S, Wahlestedt C, Walker P. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- 39.Habert Ortoli E, Amiranoff B, Loquet I, Laburthe M, Mayaux J F. Proc Natl Acad Sci USA. 1994;91:9780–9783. doi: 10.1073/pnas.91.21.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fathi Z, Cunningham A M, Iben L G, Battaglino P B, Ward S A, Nichol K A, Pine K A, Wang J C, Goldstein M E, Iismaa T P, et al. Mol Brain Res. 1997;51:49–59. doi: 10.1016/s0169-328x(97)00210-6. [DOI] [PubMed] [Google Scholar]
- 41.Howard A D, Tan C, Shiao L L, Palyha O C, McKee K K, Weinberg D H, Feighner S D, Cascieri M A, Smith R G, Van der Ploeg L H, et al. FEBS Lett. 1997;405:285–290. doi: 10.1016/s0014-5793(97)00196-8. [DOI] [PubMed] [Google Scholar]
- 42.Wang S, Hashemi T, He C, Strader C, Bayne M. Mol Pharmacol. 1997;52:337–343. doi: 10.1124/mol.52.3.337. [DOI] [PubMed] [Google Scholar]
- 43.McMahan R W, Sobel T J, Baxter M G. Hippocampus. 1997;7:130–136. doi: 10.1002/(SICI)1098-1063(1997)7:2<130::AID-HIPO2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 44.Baxter M G, Gallagher M. Behav Neurosci. 1996;110:460–467. doi: 10.1037//0735-7044.110.3.460. [DOI] [PubMed] [Google Scholar]
- 45.Leanza G, Nilsson O G, Nikkhah G, Wiley R G, Bjorklund A. Neuroscience. 1996;74:119–141. doi: 10.1016/0306-4522(96)00095-4. [DOI] [PubMed] [Google Scholar]
- 46.Kitt C A, Hohmann C, Coyle J T, Price D L. J Comp Neurol. 1994;341:117–129. doi: 10.1002/cne.903410110. [DOI] [PubMed] [Google Scholar]
- 47.Zini S, Roisin M P, Langel U, Bartfai T, Ben-Ari Y. Eur J Pharmacol. 1993;245:1–7. doi: 10.1016/0922-4106(93)90162-3. [DOI] [PubMed] [Google Scholar]
- 48.Consolo S, Baldi G, Russi G, Civenni G, Bartfai T, Vezzani A. Proc Natl Acad Sci USA. 1994;91:8047–8051. doi: 10.1073/pnas.91.17.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]