Abstract
AIMS—To assess calcarine activation with functional magnetic resonance imaging (fMRI) in patients with anisometropic and strabismic amblyopia. METHODS—14 amblyopes (eight anisometropic and six strabismic) were studied with fMRI using stimuli of checkerboards of various checker sizes and temporal frequencies. While T2* weighted MRI were obtained every 3 seconds for 6 minutes, patients viewed the stimuli monocularly with either the amblyopic or sound eye. RESULTS—Amblyopic eyes showed reduced calcarine activation compared with contralateral sound eyes in fMRI in all subjects. The calcarine activation from amblyopic eyes in anisometropic amblyopes was more suppressed at higher spatial frequencies, while that from amblyopic eyes in strabismic amblyopes was more suppressed at lower spatial frequencies. CONCLUSION—These results suggest that fMRI is a useful tool for the study of amblyopia in humans. The calcarine activation via amblyopic eyes because of anisometropia or strabismus has different temporospatial characteristics, which suggests differences in the neurophysiological mechanisms between two types of amblyopia.
Full Text
The Full Text of this article is available as a PDF (147.1 KB).
Figure 1 .
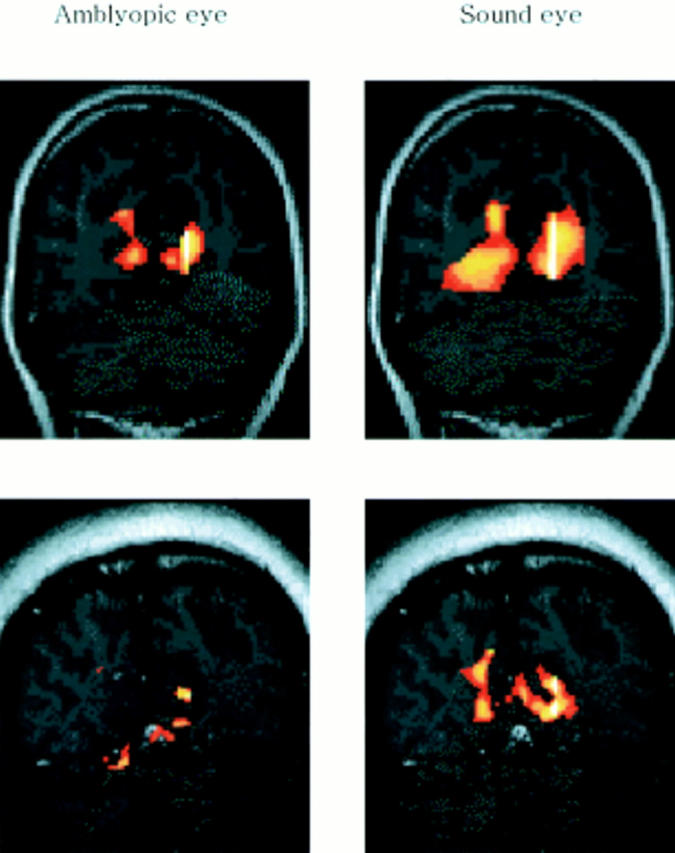
Examples of calcarine activation are shown for an anisometropic patient (upper) and a strabismic patient (lower), in which the activation by amblyopic eyes was found to be significantly less than that for the sound eyes. Images are vertically aligned along the calcarine fissures with occipital lobes. During visual stimulation, local increases in signal intensity were detected in the medial-posterior regions of the occipital lobes along the calcarine fissures. Yellow coloured regions represent a more activated state than the red coloured regions.
Figure 2 .
The average percentage change of functional MRI signal in anisometropic and strabismic amblyopias. Solid circles represent the sound eyes, open circles show the defocused response in sound eyes, and rectangles represent amblyopic eyes. Asterisks mean that the difference between the two responses was statistically significant (p<0.05).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Holliday I. E., Harding G. F. Assessment of cortical dysfunction in human strabismic amblyopia using magnetoencephalography (MEG). Vision Res. 1999 May;39(9):1723–1738. doi: 10.1016/s0042-6989(98)00259-4. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Blamire A. M., Ogawa S., Ugurbil K., Rothman D., McCarthy G., Ellermann J. M., Hyder F., Rattner Z., Shulman R. G. Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11069–11073. doi: 10.1073/pnas.89.22.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born P., Leth H., Miranda M. J., Rostrup E., Stensgaard A., Peitersen B., Larsson H. B., Lou H. C. Visual activation in infants and young children studied by functional magnetic resonance imaging. Pediatr Res. 1998 Oct;44(4):578–583. doi: 10.1203/00006450-199810000-00018. [DOI] [PubMed] [Google Scholar]
- Campos E. C., Prampolini M. L., Gulli R. Contrast sensitivity differences between strabismic and anisometropic amblyopia: objective correlate by means of visual evoked responses. Doc Ophthalmol. 1984 Aug 15;58(1):45–50. doi: 10.1007/BF00140897. [DOI] [PubMed] [Google Scholar]
- Demer J. L., Grafton S., Marg E., Mazziotta J. C., Nuwer M. Positron-emission tomographic study of human amblyopia with use of defined visual stimuli. J AAPOS. 1997 Sep;1(3):158–171. doi: 10.1016/s1091-8531(97)90059-8. [DOI] [PubMed] [Google Scholar]
- Demer J. L., von Noorden G. K., Volkow N. D., Gould K. L. Imaging of cerebral blood flow and metabolism in amblyopia by positron emission tomography. Am J Ophthalmol. 1988 Apr 15;105(4):337–347. doi: 10.1016/0002-9394(88)90294-2. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J Neurophysiol. 1984 May;51(5):1109–1120. doi: 10.1152/jn.1984.51.5.1109. [DOI] [PubMed] [Google Scholar]
- Goodyear B. G., Nicolle D. A., Humphrey G. K., Menon R. S. BOLD fMRI response of early visual areas to perceived contrast in human amblyopia. J Neurophysiol. 2000 Oct;84(4):1907–1913. doi: 10.1152/jn.2000.84.4.1907. [DOI] [PubMed] [Google Scholar]
- Headon M. P., Powell T. P. Cellular changes in the lateral geniculate nucleus of infant monkeys after suture of the eyelids. J Anat. 1973 Oct;116(Pt 1):135–145. [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A. E., Movshon J. A., Eggers H. M., Gizzi M. S., Boothe R. G., Kiorpes L. Effects of early unilateral blur on the macaque's visual system. II. Anatomical observations. J Neurosci. 1987 May;7(5):1327–1339. doi: 10.1523/JNEUROSCI.07-05-01327.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C., Hocking D. R. Pattern of ocular dominance columns in human striate cortex in strabismic amblyopia. Vis Neurosci. 1996 Jul-Aug;13(4):787–795. doi: 10.1017/s0952523800008658. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Stryker M. P. Amblyopia induced by anisometropia without shrinkage of ocular dominance columns in human striate cortex. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5494–5498. doi: 10.1073/pnas.90.12.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K., Richter H., Fischer H., Lennerstrand G., Franzén O., Rydberg A., Andersson J., Schneider H., Onoe H., Watanabe Y. Reduced activity in the extrastriate visual cortex of individuals with strabismic amblyopia. Neurosci Lett. 1997 Apr 11;225(3):173–176. doi: 10.1016/s0304-3940(97)00211-5. [DOI] [PubMed] [Google Scholar]
- Kabasakal L., Devranoğlu K., Arslan O., Erdil T. Y., Sönmezoğlu K., Uslu I., Tolun H., Isitman A. T., Ozker K., Onsel C. Brain SPECT evaluation of the visual cortex in amblyopia. J Nucl Med. 1995 Jul;36(7):1170–1174. [PubMed] [Google Scholar]
- Kiorpes L., Kiper D. C., O'Keefe L. P., Cavanaugh J. R., Movshon J. A. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998 Aug 15;18(16):6411–6424. doi: 10.1523/JNEUROSCI.18-16-06411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. K., Beason-Held L. L., Purpura K. P., Aronchick D. M., Optican L. M., Alexander G. E., Horwitz B., Rapoport S. I., Schapiro M. B. Age-related differences in visual perception: a PET study. Neurobiol Aging. 2000 Jul-Aug;21(4):577–584. doi: 10.1016/s0197-4580(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Loeffler M., Wise J. S., Gans M. Contrast sensitivity letter charts as a test of visual function in amblyopia. J Pediatr Ophthalmol Strabismus. 1990 Jan-Feb;27(1):28–31. doi: 10.3928/0191-3913-19900101-09. [DOI] [PubMed] [Google Scholar]
- Mentis M. J., Alexander G. E., Grady C. L., Horwitz B., Krasuski J., Pietrini P., Strassburger T., Hampel H., Schapiro M. B., Rapoport S. I. Frequency variation of a pattern-flash visual stimulus during PET differentially activates brain from striate through frontal cortex. Neuroimage. 1997 Feb;5(2):116–128. doi: 10.1006/nimg.1997.0256. [DOI] [PubMed] [Google Scholar]
- Noorden G. K. Mechanisms of amblyopia. Adv Ophthalmol. 1977;34:93–115. [PubMed] [Google Scholar]
- Norcia A. M., Harrad R. A., Brown R. J. Changes in cortical activity during suppression in stereoblindness. Neuroreport. 2000 Apr 7;11(5):1007–1012. doi: 10.1097/00001756-200004070-00022. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W., Noll D. C., Cohen J. D. Functional topographic mapping of the cortical ribbon in human vision with conventional MRI scanners. Nature. 1993 Sep 9;365(6442):150–153. doi: 10.1038/365150a0. [DOI] [PubMed] [Google Scholar]
- Sereno M. I., Dale A. M., Reppas J. B., Kwong K. K., Belliveau J. W., Brady T. J., Rosen B. R., Tootell R. B. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995 May 12;268(5212):889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sjöstrand J. Contrast sensitivity in children with strabismic and anisometropic amblyopia. A study of the effect of treatment. Acta Ophthalmol (Copenh) 1981 Feb;59(1):25–34. doi: 10.1111/j.1755-3768.1981.tb06706.x. [DOI] [PubMed] [Google Scholar]
- von Noorden G. K., Crawford M. L., Levacy R. A. The lateral geniculate nucleus in human anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1983 Jun;24(6):788–790. [PubMed] [Google Scholar]
- von Noorden G. K., Crawford M. L. The lateral geniculate nucleus in human strabismic amblyopia. Invest Ophthalmol Vis Sci. 1992 Aug;33(9):2729–2732. [PubMed] [Google Scholar]
- von Noorden G. K. Histological studies of the visual system in monkeys with experimental amblyopia. Invest Ophthalmol. 1973 Oct;12(10):727–738. [PubMed] [Google Scholar]
- von Noorden G. K., Middleditch P. R. Histology of the monkey lateral geniculate nucleus after unilateral lid closure and experimental strabismus: further observations. Invest Ophthalmol. 1975 Sep;14(9):674–683. [PubMed] [Google Scholar]



