Abstract
At the cerebellar synapse between the parallel fibers (PFs) and the Purkinje cells in the cerebellum, we have found that application of N-methyl-d-aspartate (NMDA) reversibly depresses the postsynaptic current. We present evidence that this depression involves NMDA receptors located on the presynaptic axons and requires that the NMDA application be combined with action potentials in the PFs. Unexpectedly, unlike other modulations mediated by presynaptic receptors, the NMDA-induced inhibition does not involve a depression of transmitter release. Because it is blocked by both nitric oxide synthase and soluble guanylate cyclase inhibitors, we propose that it involves a trans-synaptic mechanism in which NO released by the PFs decreases the glutamate sensitivity of the Purkinje cell.
At many synapses transmission is modulated by presynaptic receptors that can be either metabotropic or ionotropic. The first ionotropic receptors identified were found to activate chloride channels, but in recent years evidence has been gathered for presynaptic ionotropic receptors opening cationic channels (1). Most of the previous claims for the existence of presynaptic N-methyl-d-aspartate (NMDA) receptors were based either on observations indicating that antibodies against either the NR1 or the NR2 subunits label axons or synaptic boutons (2–8) or on functional studies suggesting a modulation by NMDA of transmitter release (9–14). However, the characterization of NMDA receptors in presynaptic terminals has been complicated by the presence of NMDA receptors on the postsynaptic side (at most glutamatergic synapses) as well as on the somatodendritic compartment of the presynaptic neuron. In searching for a glutamatergic synapse at which it would be possible to study putative presynaptic NMDA receptors in relative isolation we chose the synapse between parallel fibers (PFs) and Purkinje cells (PCs) in the cerebellar cortex. At this synapse, immunolabeling has shown the presence on PFs of both NR1 (3) and NR2 (4) subunits, and after 2 weeks of age there are no functional NMDA receptors on the postsynaptic cell (15–17). Furthermore, the well-defined organization of the cerebellar cortex permits the stimulation of a homogeneous set of glutamatergic axons and the study of the synapse PF-PC with minimal interference from the somatodendritic receptors of the presynaptic granule cells. The starting point of our study was the observation that application of NMDA on cerebellar slices depresses the PF-PC excitatory postsynaptic current (EPSC). The analysis of this effect leads us to conclude that the NMDA receptors involved were those situated on the PFs.
Methods
Thin transverse cerebellar slices (300 μm) were prepared following the method described by Llano et al. (16) from Wistar rats (aged 18–26 days). Slices were visualized by using a ×40 water-immersion objective (0.75 NA, Axioskop, Carl Zeiss) and infrared optics (illumination filter 750 ± 50 nm; Sony charge-coupled device camera).
All experiments were performed at room temperature (18–24°C). The recording chamber was continuously perfused at a rate of 1.5 ml/min with a solution containing: 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, 25 mM glucose, bubbled with 95% O2 and 5% CO2 (pH 7.4). Unless otherwise stated, strychnine (1 μM) and gabazine (20 μM) were added to the bath solution to block fast inhibitory transmission.
Patch pipettes had a resistance of 3–4.5 MΩ in solution. PCs were voltage-clamped at −70 mV in the whole-cell configuration. The internal solution contained: 120 mM CsOH, 100 mM d-gluconic acid, 20 mM tetraethylammonium-Cl, 10 mM Hepes, 5 mM QX314, 10 mM EGTA, 1 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA), 1 mM CaCl2, 5 mM MgCl2, pH adjusted to 7.2 with CsOH. Series resistance was kept between 4 and 12 MΩ; compensation was 95–98% (Axopatch 200B). Field recordings were performed with a patch pipette of 1–2 MΩ filled with Hepes-buffered saline and placed on the stimulated PF bundle. pclamp6 software (Axon Instruments, Foster City, CA) was used for data acquisition and analysis. Whole-cell recordings were filtered at 2 KHz and digitized at 10 KHz; field recordings were filtered at 10 KHz and digitized at 50 KHz.
PFs were stimulated every 20 s by means of a patch pipette filled with Hepes-buffered saline. The stimulation electrode was placed on the surface of the molecular layer at a distance of 100–500 μm from the recorded PC. Each stimulation consisted of a pair of brief pulses separated by 100 ms. Stimulation intensity was fixed (between 3 and 15 V; 30–150 μs) at the beginning of the experiment and, unless otherwise stated, remained unchanged during the experiment. In double stimulation experiments, stimulation pipettes were placed at a distance of at least 80 μm to stimulate independent axon bundles. The decay time constants of evoked synaptic currents varied from 4 to 15 ms. Stimulations in the lower part of the molecular layer produced the fastest EPSCs. To evaluate the strength of the synapse we measured the charge transferred during an 80-ms period after the stimulation. This index is less sensitive than the EPSC amplitude to the slight changes of access resistance that can occur during prolonged recordings.
NMDA, d-2-amino-5-phosphonovaleric acid (APV), 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX), 7-chlorokynurenate, ACPT-II, N(G)nitro-l-arginine methyl ester (l-NAME), and 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ) were purchased from Tocris Cookson, Bristol, U.K. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) and gabazine were from Research Biochemicals, QX314 from Alomone labs (Jerusalem, Israel), and EGTA-AM and BAPTA-AM from Molecular Probes. CGP55845A was kindly provided by Novartis. All other chemicals were from Sigma.
Results and Discussion
NMDA Receptor Activation Depresses the PF-PC Synapse.
We used the whole-cell configuration of the patch-clamp technique to record the EPSCs evoked in PCs by stimulation of the PFs in transverse slices of the rat cerebellum (Fig. 1a). When NMDA (30 μM) and glycine (10 μM) were coapplied to the bath (in the presence of 1 mM external Mg2+) the amplitude of the evoked EPSCs was reduced (Fig. 1b). After a few minutes this depression reached a maximum (42 ± 2%; n = 27). The depression was entirely reversible on washing of the agonists. EPSC kinetics remained unchanged (see Fig. 1d).
Figure 1.
Inhibition of synaptic transmission by NMDA receptors. (a) Schematic representation of a cerebellar cortex transverse slice showing the relative positions of recording and stimulating electrodes. ML: molecular layer; PCL: PC layer. (b) Effect of 30 μM NMDA + 10 μM glycine on EPSC total charge. Paired first and second pulses are represented by ○ and ●, respectively. (c) Simultaneous recordings of presynaptic volley and evoked EPSC in control conditions, after 10 min of 30 μM NMDA + 10 μM glycine and 20 min after drug washout (recovery). (d) PPF and EPSC kinetics are not affected. Traces before and during NMDA + glycine application. When the responses were scaled up to the amplitude of the first EPSC (“scaled”) (control responses: solid line; NMDA + gly: dotted line), the second EPSCs were also superimposable.
No presynaptic changes could be detected. Application of NMDA and glycine did not change the amplitude of the presynaptic volley recorded extracellularly (99 ± 2% of control value, n = 3), indicating that the stimulation excites the same number of PFs before, during, and after NMDA application (Fig. 1c). Each stimulus consisted of a pair of pulses separated by 100 ms. The second of the two successive EPSCs was always larger than the first, indicating the presence of paired pulse facilitation (PPF). PPF was characterized at this synapse by Atluri and Regehr (18) and is correlated with the residual Ca2+ transient in the PF after the first action potential. Because it is altered by presynaptic modulators affecting transmitter release (19, 20), we routinely measured it to monitor possible changes in glutamate release. PPF remained unchanged during the NMDA-induced depression (2.1 ± 0.1 vs. 2.0 ± 0.1 control ratio, n = 23; see Fig. 1d). This finding suggests that the presynaptic Ca2+ concentrations influencing transmitter release were not altered by NMDA.
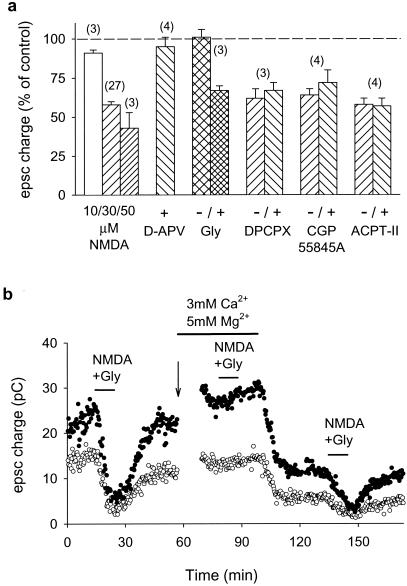
The NMDA-induced depression showed a typical NMDA receptor pharmacology (Fig. 2a). A detectable response could be induced with concentrations of NMDA as low as 10 μM, and 50 μM NMDA reduced the EPSC by more than a half. The NMDA effect was sensitive to the competitive antagonist of the glutamate site d-APV (50 μM). NMDA was unable to depress the PF-PC EPSC in the presence of a saturating concentration (33 μM) of a competitive antagonist of the NMDA receptor glycine site, 7-chlorokynurenate, and this block was reversed by 100 μM glycine. The NMDA-induced depression was reduced to 7 ± 4% (n = 4) in a solution containing 5 mM Mg2+ and reappeared as soon as the external Mg2+ concentration was returned to 1 mM (Fig. 2b).
Figure 2.
Pharmacological characterization of the NMDA-induced depression of synaptic transmission. (a) Bar chart shows the effects (measured after 10 min of agonist application) of three different concentrations of NMDA in the presence of 10 μM glycine; 30 μM NMDA + 10 μM glycine in the presence of 50 μM d-APV; 30 μM NMDA with or without 100 μM glycine in the presence of 33 μM 7-chlorokynurenate; 30 μM NMDA + 10 μM glycine in the presence or the absence of 200 nM DPCPX, 5 μM CGP55845A, or 100 μM ACPT-II. Data represent mean ± SEM of n experiments (numbers in parentheses). (b) The NMDA-induced depression was sensitive to high extracellular Mg2+ (5 mM) and reappeared as soon as the Mg2+ concentration was returned to 1 mM. Paired first and second pulses are represented by ○ and ●, respectively. The arrow indicates a decrease in stimulation strength to obtain EPSCs of similar amplitude.
Presynaptic Location of the NMDA Receptors Responsible for the Synaptic Depression.
The depression of the PF-PC synapse induced by NMDA does not depend on postsynaptic NMDA receptors located in PCs. Functional NMDA receptors have been reported to disappear from the PC after 2 weeks of age (15, 16) and indeed, no current was evoked in the PC by the bath application of up to 50 μM NMDA and 10 μM glycine (in the presence of gabazine and strychnine) in the 18- to 26-day-old animals used in our experiments.
The NMDA-induced depression of the PF-PC synapse is not caused by NMDA receptors located on the soma or the dendrites of granule cells. The depression could still be observed (35 ± 5%; n = 4) when the NMDA application (50 μM NMDA and 10 μM glycine) was restricted to the molecular layer over the recorded PC (20 μM d-APV was added to the bath to block the NMDA receptors not directly exposed to the local NMDA perfusion).
Interneurons of the molecular layer are active in the slices and they express NMDA receptors. In various preparations bath-applied NMDA has been found to induce the release by interneurons of various transmitters modulating glutamate release, and this possibility had to be considered with particular attention because recordings made in the PCs during the application of NMDA showed a marked increase in the frequency of inhibitory postsynaptic currents, indicating an increased firing of interneurons. Furthermore, the two transmitters that have been found in other preparations to mediate indirect effects of NMDA, adenosine and γ-aminobutyric acid (GABA) (21, 22), are present in the cerebellar cortex and are known to modulate transmitter release at the PF-PC synapse through GABA-B and adenosine A1 receptors (20, 23). We therefore tested the effect of NMDA in the presence of potent and specific inhibitors of these two receptors. The NMDA effect was blocked neither by the high-affinity GABA-B receptor antagonist CGP55845A nor by the high-affinity adenosine A1 receptor antagonist DPCPX (Fig. 2a). More generally, the presynaptic modulations described in the literature (20–23) all appear to involve changes in transmitter release probability, and in the cerebellum they alter PPF (20, 23). This contrasts with the fact that, as shown in Fig. 1, the NMDA-induced depression of synaptic strength does not alter this form of presynaptic plasticity. Inhibition of metabotropic glutamate receptors by application of 100 μM ACPT-II did not affect the NMDA-induced depression of the PF-PC synapse.
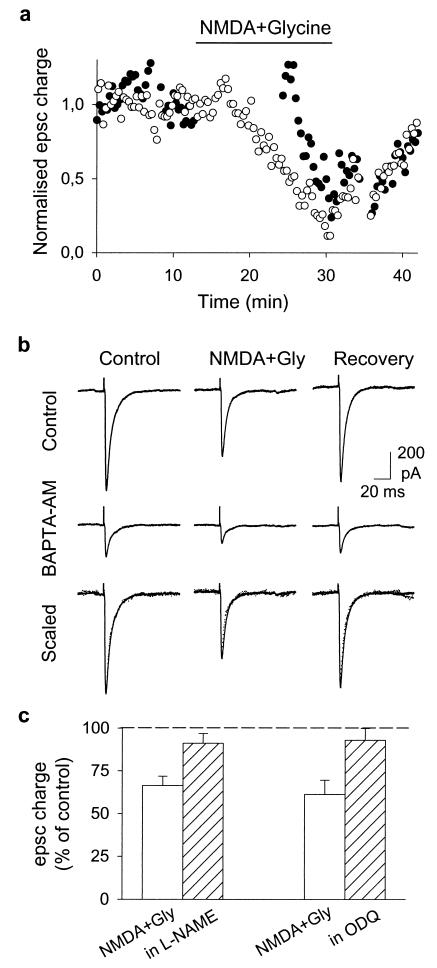
Nevertheless, we could not immediately exclude the possibility that the interneurons excited by bath applied NMDA release unidentified mediators (other than adenosine or γ-aminobutyric acid) able to act on the PFs or PCs. We ruled out this possibility with experiments showing that the application of NMDA is not itself sufficient to depress the PF-PC synapse, although interneurons are strongly excited. The synaptic depression requires coincidence of NMDA application with action potentials in the PFs. In the experiments showing this, two stimulating electrodes were placed at different heights in the molecular layer and alternate EPSCs were evoked in the same PC at an interval of 10 s (Fig. 3a). When NMDA was applied, stimulation at one of the locations was discontinued and the other was maintained. The depression developed for the EPSC for which the stimulation was maintained and reached 39 ± 2% (n = 6) 10 min after the beginning of the NMDA application. The stimulation of the second pathway then was resumed, and it was found that the first EPSC elicited by this stimulation had not been reduced by the NMDA application (1 ± 8% inhibition; n = 6). The following EPSCs evoked by the second pathway then started to decline and soon reached a level similar to that of the first pathway. We can conclude that the inhibition requires the conjunction of NMDA application and PF action potentials.
Figure 3.
Mechanism of the NMDA-induced depression of synaptic transmission. (a) Effect of application of 30 μM NMDA + 10 μM glycine on EPSCs evoked by two different stimulation electrodes. Stimulation 1 (○) was applied every 20 s during the whole experiment. Stimulation 2 (●) was applied at the same frequency but was stopped immediately before agonist application and resumed 10 min later. EPSC charge is normalized to the mean value of the period preceding agonist application (stimulation 1: 4.2 pC; stimulation 2: 5.7 pC). Note that as few as 30 action potentials in the presence of NMDA are sufficient to induce a 50% decrease of the PF EPSC. (b) Reducing glutamate release by incubating the slice in BAPTA-AM (50 μM, 5 min) reduced the PF-PC EPSC but did not modify the effect of NMDA. When the responses recorded after BAPTA-AM treatment and in the absence of NMDA were scaled up to the amplitude of the control EPSCs (“scaled”) (control responses: solid line; BAPTA-AM: dotted line), the responses recorded in the presence of NMDA scaled by the same factor were also superimposable. (c) Effect of 30 μM NMDA + 10 μM glycine application on EPSC charge in control conditions (empty bars) or in the presence of l-NAME (1 mM) or ODQ (1 μM) (hatched bars). Bars represent means ± SEM of three experiments.
The simplest explanation of these observations is that in the absence of action potentials the Mg2+ block prevents the opening by NMDA of the PF NMDA receptors. The requirement for PF action potentials is also consistent with the report by Glitsch and Marty (13) that at the PF-PC synapse the size of the spontaneous (miniature) EPSCs is not changed by an NMDA application. More generally, our observations and those of Glitsch and Marty indicate that interneurons activated by the application of NMDA cannot be the source of an unknown compound that would diffuse through the slice and modulate the PF-PC synapse. However, a residual hypothesis had to be considered, namely that the relief of the Mg2+ block could occur on NMDA receptors situated on interneurons contacted by the active PFs. These interneurons would not be activated by the application of NMDA alone, but would be depolarized if the NMDA application combined its effects with the activation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by the glutamate released by the PF action potential. The hypothesis appeared somewhat unlikely because it implies that the interneurons involved should be specifically associated with the PF-PC synapses activated by the PF stimulation. Nevertheless, we decided to test one of its predictions, namely that a reduction in glutamate release should interfere with the NMDA-induced depression of the PF-PC synapse. To depress transmitter release we loaded the PFs with Ca2+ chelators by incubating the whole slice with EGTA-AM or BAPTA-AM (50 μM, 5 min). After treatment of the slice with EGTA-AM the EPSC was substantially depressed (50 ± 9%) and the PPF disappeared (from 2.0 ± 0.1 to 1.1 ± 0.1; n = 3). Nevertheless, the NMDA-induced depression remained essentially unchanged (44 ± 3% with EGTA-AM, control value 44 ± 6%; n = 3). Similar results were obtained with BAPTA-AM (Fig. 3b).
The effects of membrane-permeable Ca2+ chelators, when considered together with the fact that the NMDA-induced depression requires PF action potentials, strongly support the claim that the NMDA receptors involved in the PF-PC synaptic depression are those situated on the PFs and not those situated on the molecular layer interneurons.
The lack of effect of Ca2+ chelators on the NMDA-induced depression points out the existence of two different Ca2+ microdomains, with the Ca2+ pathway responsible for transmitter release being more sensitive to chelators than the one presumably involved in the NMDA-induced depression. This difference could be accounted for by different distances between the sites of Ca2+ entry and the Ca2+ sensors, with NMDA receptors being closer to their targets than Ca2+ channels to theirs. The hypothesis of two different Ca2+ microdomains also would account for the lack of changes in transmitter release on NMDA application, as determined from PPF measurements. We set out to characterize the mechanism of the NMDA-induced depression, because identification of the Ca2+-sensitive detectors involved could provide further information regarding its Ca2+ dependence.
Role of NO in the NMDA-Induced Depression.
How does the activation of presynaptic NMDA receptors lead to a decrease in the size of the EPSC? Propagation block was not detected (Fig. 1c), and any depression of transmitter release would be difficult to reconcile with the absence of changes in the PPF (Fig. 1d). A postsynaptic expression of the phenomenon thus appeared more likely, but required a trans-synaptic messenger. In the search for such a messenger, we took into account reports of the presence of NO synthase (NOS) in PFs (24), of NO production by these fibers (25), and of a body of observations suggesting that NO released by the PFs can activate the soluble guanylate cyclase (sGC) expressed by the PCs (26, 27) and trigger a postsynaptic decrease in the glutamate sensitivity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. This trans-synaptic signaling pathway has been most systematically described in the analysis of cerebellar long-term depression (ref. 28, for a review see ref. 19), but it also is reported to be involved in a transient depression at this synapse (29). Because Ca2+ influx through NMDA receptors is known to activate the NOS through a Ca-calmodulin complex (ref. 30, and for a review see ref. 31), we tested the hypothesis that the NMDA-induced depression of the PF-PC synapse could involve NO. Indeed, after incubation of the slice with either 1 mM l-NAME (an inhibitor of the NOS) or 1 μM ODQ (a potent and specific inhibitor of the sGC), NMDA application was no longer able to depress the PF-PC synapse (Fig. 3c). These results suggest a sequence of events in which action potential-dependent unblocking of the PF NMDA receptors channels would induce a local (and transient) increase of PF calcium. This Ca2+ entry would activate the PF NOS, and diffusion of NO to the PC would activate the sGC, triggering a decrease of the postsynaptic sensitivity to glutamate.
In this model the Ca2+ leading to NOS activation enters through a pathway (NMDA receptors) separate from that used by the Ca2+ leading to transmitter release (voltage-dependent Ca2+ channels). In other systems it has been shown that the NOS activation involves high-affinity Ca2+ binding, probably through calmodulin (31) whereas transmitter release involves a relatively lower affinity Ca2+ binding (32–34). Despite this difference of affinities, Ca2+ buffering with EGTA-AM or BAPTA-AM did not affect the NOS-dependent process at concentrations that reduced by half transmitter release. This observation is in accordance with reports showing that concentrations of a few mM of Ca2+ chelators substantially reduced transmitter release (32, 33) but had little effect on processes depending on Ca-calmodulin (35, 36). This disparity can be explained by different distances between the sites of Ca2+ entry and the Ca2+ sensors, with NMDA receptors being closer to NOS than Ca2+ channels to the Ca2+ sensor for transmitter release. This hypothesis is supported by the tight and selective coupling between NMDA receptors and NOS through interactions with some of the MAGUKs (membrane-associated guanylate kinases) family proteins (30, 31).
If NOS is a relatively high-affinity target for Ca2+, could Ca2+ entering through voltage-dependent channels diffuse to this site and depress the synaptic current? This does not seem to be the case at low frequencies of PF activity, because no depression of the synaptic current is observed in the absence of NMDA. This finding suggests that the two pathways of Ca2+ entry (NMDA receptors and voltage-dependent Ca2+ channels) lead to two spatially separated Ca2+ “microdomains” (37), which do not impinge on each other at low frequencies of PF activity. In such conditions, Ca2+ entering through NMDA channels would be the physiological activator of NOS. This hypothesis does not exclude that at higher frequencies NOS also may be activated by an “internal spillover” of Ca2+ entering through the voltage-dependent Ca2+ channels (25).
In physiological conditions, the presynaptic NMDA receptors must be activated by an increase of extracellular glutamate around the “active” PF varicosities. Such an increase could be caused by glutamate released from the PF themselves during a period of repetitive activity, but it also could result from the reverse activity of the glutamate transporters present in Bergmann glial cells (38) or from glutamate released from the PCs themselves (39, 40). In such cases, the PF-PC synapse could exhibit plasticity even if active at extremely low frequencies.
Acknowledgments
We thank B. Barbour, J. Kehoe, and J. Neyton for critical comments on the manuscript. This work was supported by the Centre National de la Recherche Scientifique (Unité Mixte de Recherche 8544). M.C. was supported by an Ecole Supérieure de Physique et de Chimie Industrielles contract and a fellowship from the Spanish Ministry of Education and Culture.
Abbreviations
- NMDA
N-methyl-d-aspartate
- PF
parallel fiber
- PC
Purkinje cell
- EPSC
excitatory postsynaptic current
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate
- APV
2-amino-5-phosphonovaleric acid
- l-NAME
N(G)nitro-l-arginine methyl ester
- ODQ
1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- PPF
paired pulse facilitation
- NOS
NO synthase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.200354297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.200354297
References
- 1.MacDermott A B, Role L W, Siegelbaum S. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Wang H, Sheng M, Jan L Y, Jan Y N, Basbaum A I. Proc Natl Acad Sci USA. 1994;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petralia R S, Yokotani N, Wenthold R J. J Neurosci. 1994;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petralia R S, Wang Y-X, Wenthold R J. J Neurosci. 1994;14:6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conti F, Minelli A, Debiasi S, Melone M. Mol Neurobiol. 1997;14:1–18. doi: 10.1007/BF02740618. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel A, Benke D, Mohler H, Fritschy J M. Neuroscience. 1997;78:1105–1112. doi: 10.1016/s0306-4522(96)00663-x. [DOI] [PubMed] [Google Scholar]
- 7.Carlton S M, Chung K, Ding Z, Coggeshall R E. Neuroscience. 1998;83:601–605. doi: 10.1016/s0306-4522(97)00406-5. [DOI] [PubMed] [Google Scholar]
- 8.Feinstein N, Parnas D, Parnas H, Dudel J, Parnas I. J Neurophysiol. 1998;80:2893–2899. doi: 10.1152/jn.1998.80.6.2893. [DOI] [PubMed] [Google Scholar]
- 9.Parnas H, Parnas I, Ravin R, Yudelevitch B. Proc Natl Acad Sci USA. 1994;91:11586–11590. doi: 10.1073/pnas.91.24.11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu W, Liou J, Lee Y, Liou H. J Physiol (London) 1995;489:813–823. doi: 10.1113/jphysiol.1995.sp021094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berretta N, Jones R S G. Neuroscience. 1996;75:339–344. doi: 10.1016/0306-4522(96)00301-6. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Mantyh P W, Basbaum A I. Nature (London) 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 13.Glitsch M, Marty A. J Neurosci. 1999;19:511–519. doi: 10.1523/JNEUROSCI.19-02-00511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochilla A J, Alford S T. J Neurosci. 1999;19:193–205. doi: 10.1523/JNEUROSCI.19-01-00193.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrant M, Cull-Candy S G. Proc R Soc London Ser B. 1991;244:179–184. doi: 10.1098/rspb.1991.0067. [DOI] [PubMed] [Google Scholar]
- 16.Llano I, Marty A, Armstrong C M, Konnerth A. J Physiol (London) 1991;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Momiyama A, Feldmeyer D, Cull-Candy S G. J Physiol (London) 1996;494:479–492. doi: 10.1113/jphysiol.1996.sp021507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atluri P P, Regehr W G. J Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniel H, Levenes C, Crepel F. Trends Neurosci. 1998;21:401–407. doi: 10.1016/s0166-2236(98)01304-6. [DOI] [PubMed] [Google Scholar]
- 20.Dittman J S, Regehr W G. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manzoni O J, Manabe T, Nicoll R A. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- 22.Harvey J, Lacey M G. J Neurosci. 1997;17:5271–5280. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kocsis J D, Eng D L, Bhisitkul R B. Proc Natl Acad Sci USA. 1984;81:6531–6534. doi: 10.1073/pnas.81.20.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bredt D S, Glatt C E, Hwang P M, Fotuhi M, Dawson T M, Snyder S H. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- 25.Shibuki K, Kimura S. J Physiol (London) 1997;498:443–452. doi: 10.1113/jphysiol.1997.sp021870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ariano M A, Lewicki J A, Brandwein H J, Murad F. Proc Natl Acad Sci USA. 1982;79:1316–1320. doi: 10.1073/pnas.79.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boxall A R, Garthwaite J. Eur J Neurosci. 1996;8:2209–2212. doi: 10.1111/j.1460-9568.1996.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 28.Lev-Ram V, Makings L R, Keitz P F, Kao J P Y, Tsien R Y. Neuron. 1995;15:407–415. doi: 10.1016/0896-6273(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 29.Hartell N A. J Neurosci. 1996;16:2881–2890. doi: 10.1523/JNEUROSCI.16-09-02881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sattler R, Xiong Z, Lu W-Y, Hafner M, MacDonald J F, Tymianski M. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 31.Brenman J E, Bredt D S. Curr Opin Neurobiol. 1997;7:374–378. doi: 10.1016/s0959-4388(97)80065-7. [DOI] [PubMed] [Google Scholar]
- 32.Neher E, Augustine G J. J Physiol (London) 1992;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohana O, Sakmann B. J Physiol (London) 1998;513:135–148. doi: 10.1111/j.1469-7793.1998.135by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borst J G G, Helmchen F, Sakmann B. J Physiol (London) 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenmund C, Westbrook G L. J Physiol (London) 1993;470:705–729. doi: 10.1113/jphysiol.1993.sp019884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medina I, Leinekugel X, Ben-Ari Y. Eur J Neurosci. 1999;11:2422–2430. doi: 10.1046/j.1460-9568.1999.00664.x. [DOI] [PubMed] [Google Scholar]
- 37.Simon S M, Llinas R. Biophys J. 1985;48:485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark B A, Barbour B. J Physiol (London) 1997;502:335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi M, Sarantis M, Attwell D. J Physiol (London) 1996;497:523–530. doi: 10.1113/jphysiol.1996.sp021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glitsch M, Llano I, Marty A. J Physiol (London) 1996;497:531–537. doi: 10.1113/jphysiol.1996.sp021786. [DOI] [PMC free article] [PubMed] [Google Scholar]