Abstract
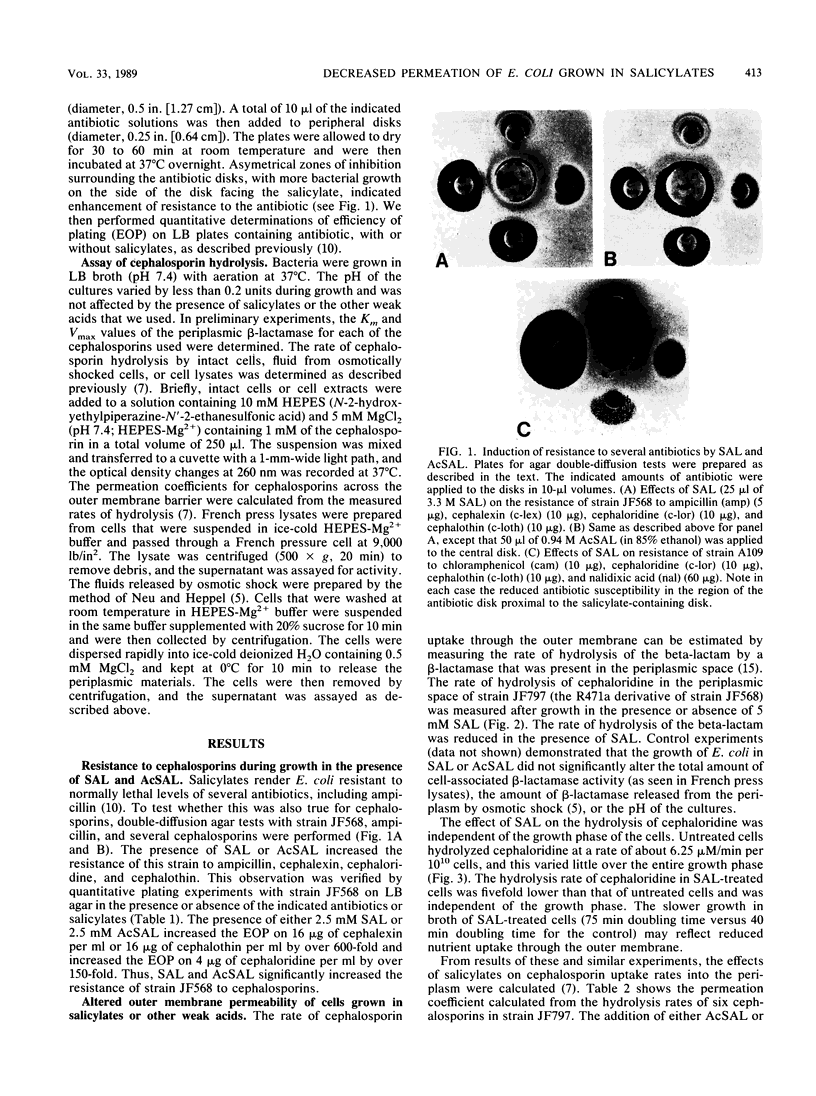
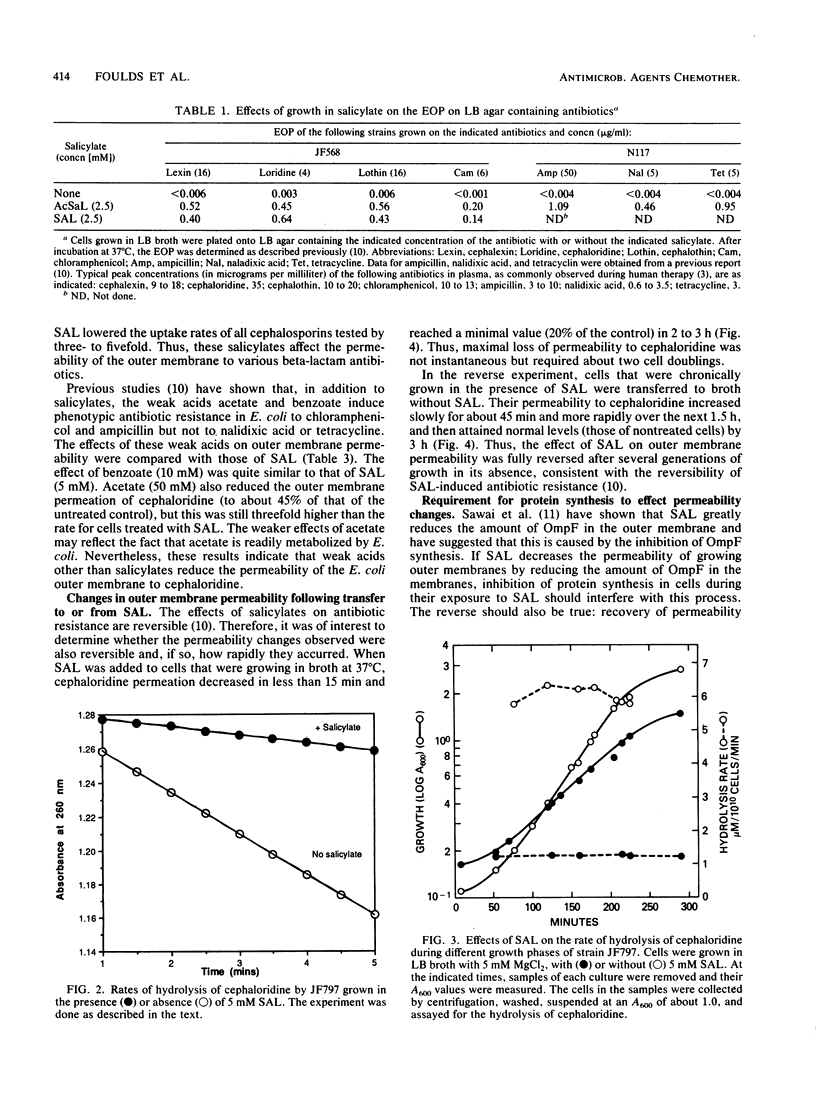
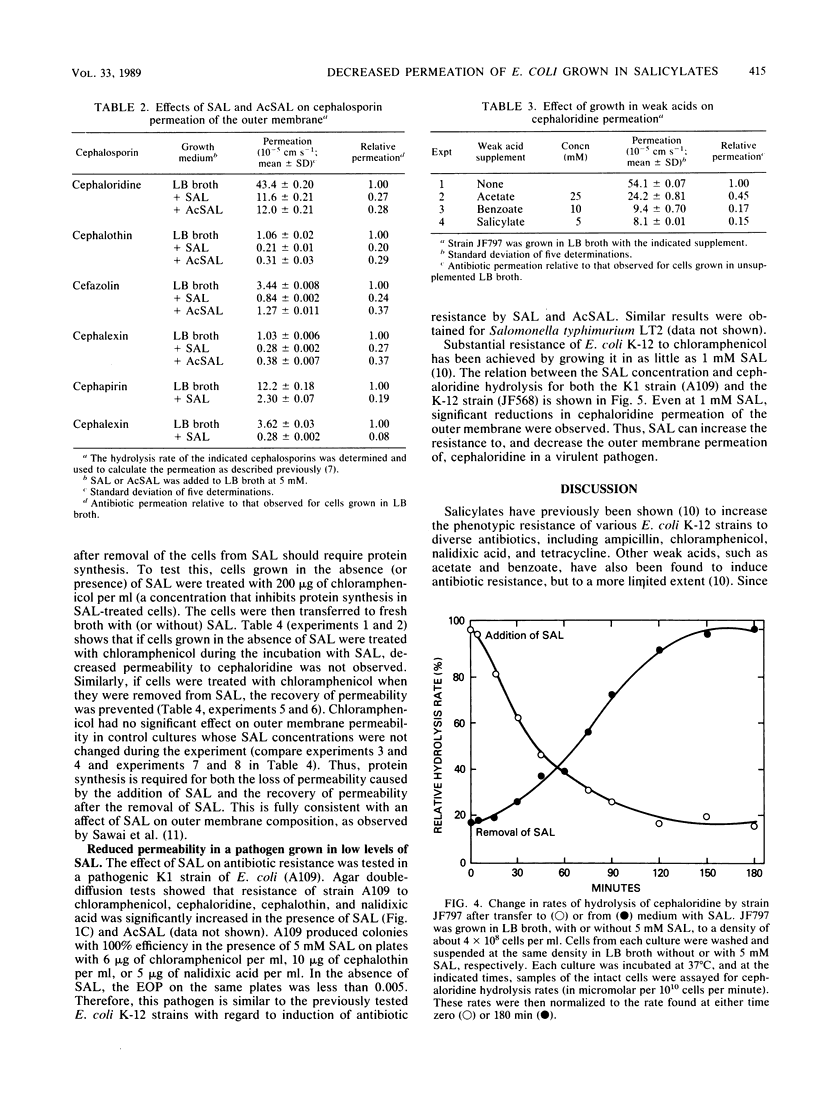
Escherichia coli K-12 cells grown in 1 to 5 mM sodium salicylate (SAL) or acetylsalicylate show increased phenotypic resistance to various antibiotics (J. L. Rosner, Proc. Natl. Acad. Sci. USA 82:8771-8774, 1985), including cephalosporins (this study). To determine whether these effects are caused by a decreased uptake of the antibiotics, the permeation of several cephalosporins through the outer membrane was measured. For E. coli K-12 grown in LB broth containing 5 mM SAL or acetylsalicylate, permeation of the outer membrane by the five cephalosporins tested decreased three- to fivefold compared with that in cells not grown in salicylates. Permeation of the outer membrane by cephaloridine decreased within 15 min of the addition of SAL to cells grown in broth and reached a minimum in 1 to 2 h. When cells were transferred from broth with SAL to broth without SAL, their permeability to cephaloridine increased slowly for the first 45 min and more rapidly over the next 1.5 h; the permeability then attained normal levels by 3 h. The permeability changes that occurred after media shifts, either to or from SAL, were prevented by concentrations of chloramphenicol that inhibited protein synthesis. These effects of SAL on outer membrane permeability are fully consistent with their effects on antibiotic resistance and with the report (T. Sawai, S. Hirano, and A. Yamaguchi, FEMS Microbiol. Lett. 40:233-237, 1987) that the outer membranes of SAL-treated cells are deficient in certain porins. Permeation of cephaloridine through the outer membrane also decreased when a virulent strain of E. coli K1 was grown in the presence of as little as 1 to 2 mM SAL. This raises the concern that high levels of salicylates in patients night interfere with cephalosporin or other antibiotic therapies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Escherichia coli K-12 tolF mutants: alterations in protein composition of the outer membrane. J Bacteriol. 1977 May;130(2):781–786. doi: 10.1128/jb.130.2.781-786.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nikaido H. Role of permeability barriers in resistance to beta-lactam antibiotics. Pharmacol Ther. 1985;27(2):197–231. doi: 10.1016/0163-7258(85)90069-5. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Nonheritable resistance to chloramphenicol and other antibiotics induced by salicylates and other chemotactic repellents in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8771–8774. doi: 10.1073/pnas.82.24.8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Aaronson W., Sutton A., Schneerson R. Comparative analysis of plasmids and some metabolic characteristics of Escherichia coli K1 from diseased and healthy individuals. Infect Immun. 1980 Jul;29(1):200–206. doi: 10.1128/iai.29.1.200-206.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski J. L., Gonzalez T. N., Bartholomew F. M., Holt N. J. Mu d-directed lacZ fusions regulated by low pH in Escherichia coli. J Bacteriol. 1987 Jul;169(7):3001–3006. doi: 10.1128/jb.169.7.3001-3006.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]