Abstract
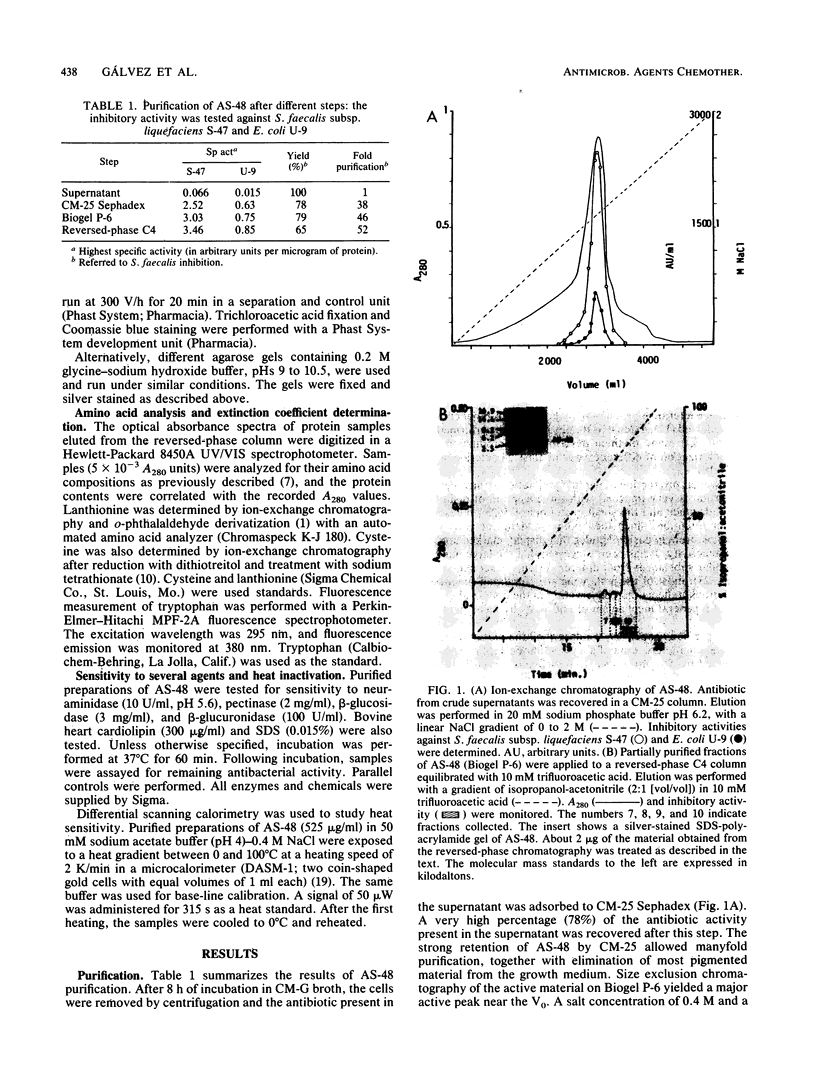
Peptide antibiotic AS-48 was purified to homogeneity by ion-exchange chromatography, gel filtration chromatography, and reversed-phase liquid chromatography. The purified fraction was active against gram-positive and gram-negative bacteria. AS-48 is a basic protein with an isoelectric point of ca. 10.5 and a molecular mass of 7.4 kilodaltons. Its inhibitory activity was markedly affected by sodium dodecyl sulfate and cardiolipin but not by neuraminidase, pectinase, beta-glucosidase, or beta-glucuronidase. Differential scanning calorimetry data suggested that AS-48 molecules lack a compact structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D., PEACHER B., PIERSON D. SURVEY OF THE BACTERIOCINES OF ENTEROCOCCI. J Bacteriol. 1963 Oct;86:702–707. doi: 10.1128/jb.86.4.702-707.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. R., Hare P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):619–622. doi: 10.1073/pnas.72.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson D. M., Tew J. G. beta-Lysin of platelet origin. Bacteriol Rev. 1977 Jun;41(2):501–513. doi: 10.1128/br.41.2.501-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Galvez A., Valdivia E., Maqueda M., Montoya E. Production of bacteriocin-like substances by group D streptococci of human origin. Microbios. 1985;43(176S):223–232. [PubMed] [Google Scholar]
- Gimenez-Gallego G., Thomas K. A. High-performance liquid chromatography of phenylthiocarbamyl-amino acids. Application to carboxyl-terminal sequencing of proteins. J Chromatogr. 1987 Nov 13;409:299–304. doi: 10.1016/s0021-9673(01)86806-0. [DOI] [PubMed] [Google Scholar]
- Gálvez A., Maqueda M., Valdivia E., Quesada A., Montoya E. Characterization and partial purification of a broad spectrum antibiotic AS-48 produced by Streptococcus faecalis. Can J Microbiol. 1986 Oct;32(10):765–771. doi: 10.1139/m86-141. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Iwanami T., Hirasawa M., Watanabe C., McGhee J. R., Shiota T. Purification and certain properties of a bacteriocin from Streptococcus mutans. Infect Immun. 1982 Mar;35(3):861–868. doi: 10.1128/iai.35.3.861-868.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis A. S., Liu T. Y. The stability of cysteine and cystine during acid hydrolysis of proteins and peptides. J Biol Chem. 1970 Jan 10;245(1):112–116. [PubMed] [Google Scholar]
- KJEMS E. Studies on streptococcal bacteriophages. I. Technique of isolating phage-producing strains. Acta Pathol Microbiol Scand. 1955;36(5):433–440. [PubMed] [Google Scholar]
- Krämer J., Brandis H. Mode of action of two streptococcus faecium bacteriocins. Antimicrob Agents Chemother. 1975 Feb;7(2):117–120. doi: 10.1128/aac.7.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Matsuo Y. Bacteriocin and hemolysin from Streptococcus faecium. Antimicrob Agents Chemother. 1981 Oct;20(4):542–544. doi: 10.1128/aac.20.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Rosenthal S. L., Buchanan A. M. Influences of cationic bactericidal agents on membrane ATPase of Bacillus subtilis. Biochim Biophys Acta. 1974 Sep 6;363(2):141–150. doi: 10.1016/0005-2736(74)90054-6. [DOI] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G. Bactericidal cationic peptides involved in bacterial antagonism and host defence. Microbiol Sci. 1985 Jul;2(7):212–217. [PubMed] [Google Scholar]
- Sahl H. G., Brandis H. Production, purification and chemical properties of an antistaphylococcal agent produced by Staphylococcus epidermidis. J Gen Microbiol. 1981 Dec;127(2):377–384. doi: 10.1099/00221287-127-2-377. [DOI] [PubMed] [Google Scholar]
- Sahl H. G., Grossgarten M., Widger W. R., Cramer W. A., Brandis H. Structural similarities of the staphylococcin-like peptide Pep-5 to the peptide antibiotic nisin. Antimicrob Agents Chemother. 1985 May;27(5):836–840. doi: 10.1128/aac.27.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G. Influence of the staphylococcinlike peptide Pep 5 on membrane potential of bacterial cells and cytoplasmic membrane vesicles. J Bacteriol. 1985 May;162(2):833–836. doi: 10.1128/jb.162.2.833-836.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Ikeda T., Mitsui I., Shiota T. Mode of inhibitory action of a bacteriocin produced by Streptococcus mutans C3603. Infect Immun. 1984 May;44(2):370–378. doi: 10.1128/iai.44.2.370-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil R., Scheit K. H. Amino acid sequence of seminalplasmin, an antimicrobial protein from bull semen. EMBO J. 1983;2(7):1159–1163. doi: 10.1002/j.1460-2075.1983.tb01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]



