Abstract
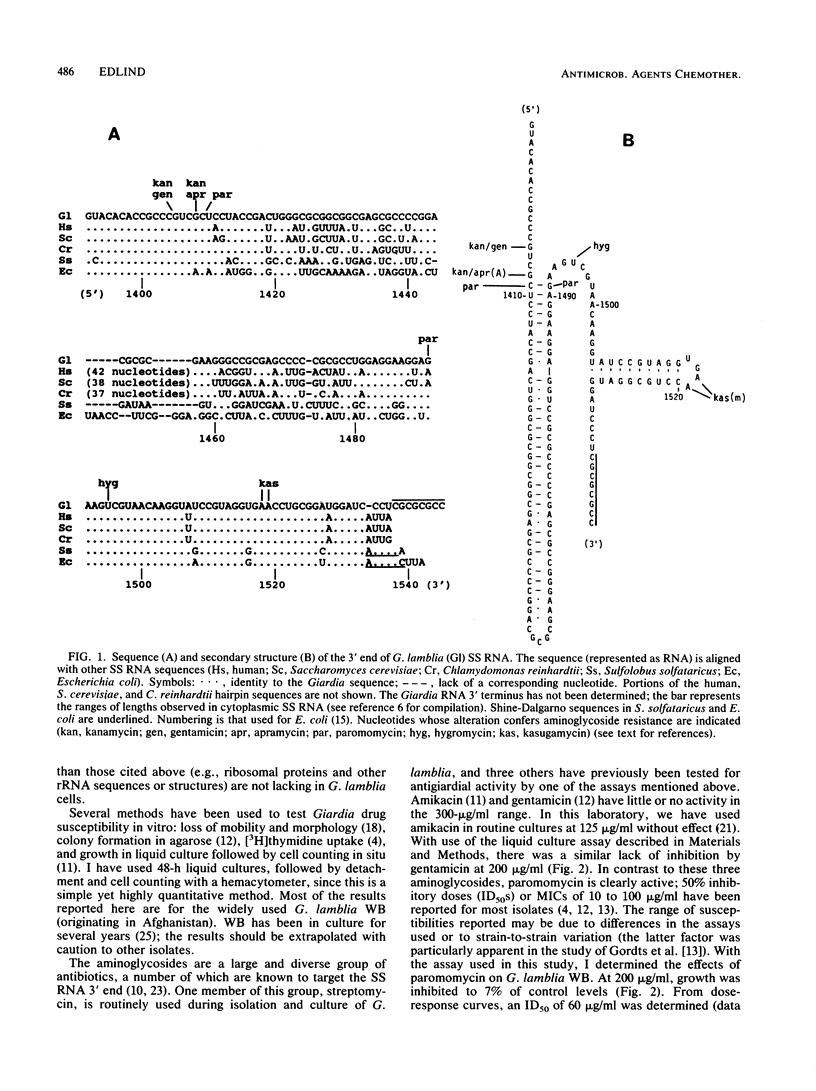
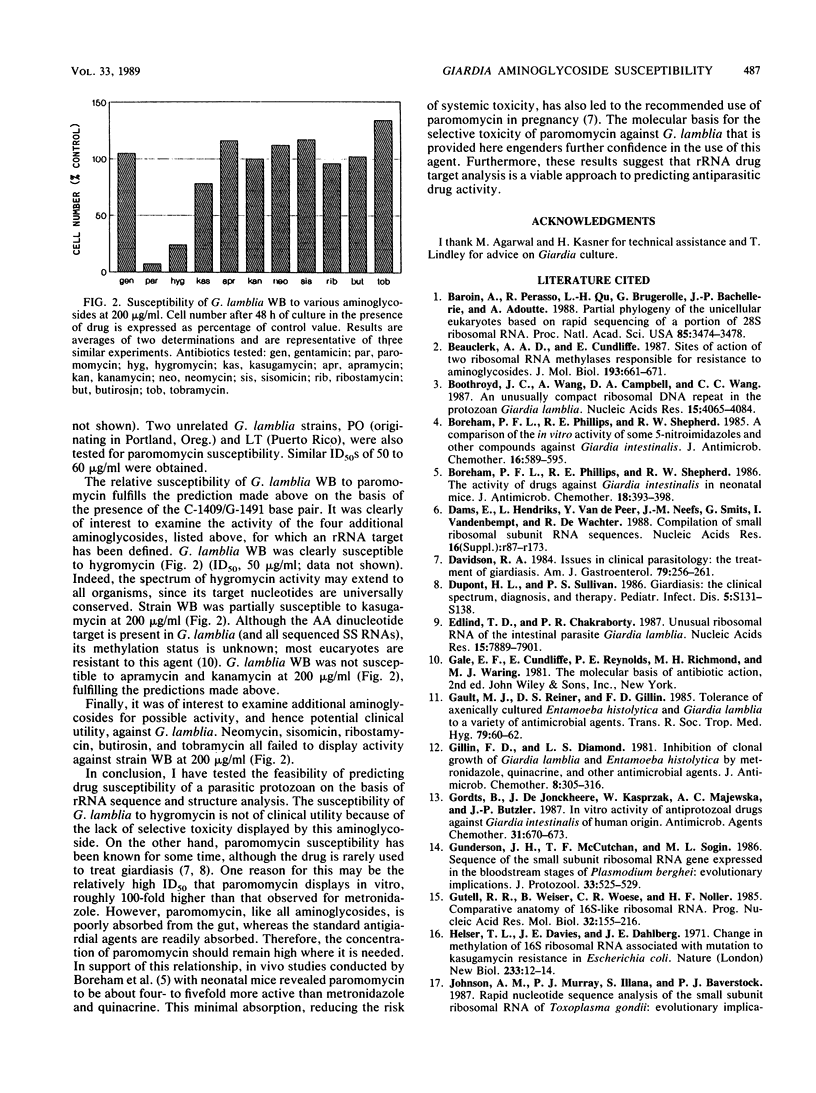
The very limited development of antiparasitic agents targeting protein synthesis stems in part from the belief that parasite and host ribosomes are sufficiently similar to preclude selective toxicity. However, recent studies have revealed that Giardia lamblia rRNA has an unusual size and sequence; consequently, this organism and its homogeneous rRNA provide a useful model for the development of protein synthesis inhibitors with antiparasitic activity. In this study, I determined the sequence and secondary structure of the 3' end of the small-subunit RNA, the target for aminoglycoside inhibitory activity. The primary structure of these 140 nucleotides includes two blocks of sequence highly conserved among other organisms; the remaining sequence, although not conserved, can be folded into a secondary structure common to all rRNAs. The presence of U-1495 within one of the conserved blocks predicts hygromycin susceptibility. Also, a specific base pair (C-1409.G-1491) implicated in paromomycin susceptibility is present; whereas all procaryotes have this base pair, it is absent in many eucaryotes (including mammals). Conversely, kanamycin and apramycin resistance can be predicted from substitution of A-1408 with G. A growth inhibition assay was used to test the susceptibility of G. lamblia to a variety of aminoglycosides. After 48 h, 8 of 11 aminoglycosides tested failed to inhibit growth at a concentration of 200 micrograms/ml. Paromomycin and hygromycin, however, inhibited growth of three strains tested by 50% at 50 to 60 micrograms/ml and by close to 90% at 120 micrograms/ml. These results correlate well with the sequence and secondary-structure analyses. Paromomycin may be clinically useful when the toxicity of standard antigiardial drugs is of concern.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroin A., Perasso R., Qu L. H., Brugerolle G., Bachellerie J. P., Adoutte A. Partial phylogeny of the unicellular eukaryotes based on rapid sequencing of a portion of 28S ribosomal RNA. Proc Natl Acad Sci U S A. 1988 May;85(10):3474–3478. doi: 10.1073/pnas.85.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauclerk A. A., Cundliffe E. Sites of action of two ribosomal RNA methylases responsible for resistance to aminoglycosides. J Mol Biol. 1987 Feb 20;193(4):661–671. doi: 10.1016/0022-2836(87)90349-4. [DOI] [PubMed] [Google Scholar]
- Boothroyd J. C., Wang A., Campbell D. A., Wang C. C. An unusually compact ribosomal DNA repeat in the protozoan Giardia lamblia. Nucleic Acids Res. 1987 May 26;15(10):4065–4084. doi: 10.1093/nar/15.10.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boreham P. F., Phillips R. E., Shepherd R. W. A comparison of the in-vitro activity of some 5-nitroimidazoles and other compounds against Giardia intestinalis. J Antimicrob Chemother. 1985 Nov;16(5):589–595. doi: 10.1093/jac/16.5.589. [DOI] [PubMed] [Google Scholar]
- Boreham P. F., Phillips R. E., Shepherd R. W. The activity of drugs against Giardia intestinalis in neonatal mice. J Antimicrob Chemother. 1986 Sep;18(3):393–398. doi: 10.1093/jac/18.3.393. [DOI] [PubMed] [Google Scholar]
- Dams E., Hendriks L., Van de Peer Y., Neefs J. M., Smits G., Vandenbempt I., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1988;16 (Suppl):r87–173. doi: 10.1093/nar/16.suppl.r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. A. Issues in clinical parasitology: the treatment of giardiasis. Am J Gastroenterol. 1984 Apr;79(4):256–261. [PubMed] [Google Scholar]
- Dupont H. L., Sullivan P. S. Giardiasis: the clinical spectrum, diagnosis and therapy. Pediatr Infect Dis. 1986 Jan-Feb;5(1 Suppl):S131–S138. [PubMed] [Google Scholar]
- Edlind T. D., Chakraborty P. R. Unusual ribosomal RNA of the intestinal parasite Giardia lamblia. Nucleic Acids Res. 1987 Oct 12;15(19):7889–7901. doi: 10.1093/nar/15.19.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault M. J., Reiner D. S., Gillin F. D. Tolerance of axenically cultured Entamoeba histolytica and Giardia lamblia to a variety of antimicrobial agents. Trans R Soc Trop Med Hyg. 1985;79(1):60–62. doi: 10.1016/0035-9203(85)90236-6. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Diamond L. S. Inhibition of clonal growth of Giardia lamblia and Entamoeba histolytica by metronidazole, quinacrine, and other antimicrobial agents. J Antimicrob Chemother. 1981 Oct;8(4):305–316. doi: 10.1093/jac/8.4.305. [DOI] [PubMed] [Google Scholar]
- Gordts B., De Jonckheere J., Kasprzak W., Majewska A. C., Butzler J. P. In vitro activity of antiprotozoal drugs against Giardia intestinalis of human origin. Antimicrob Agents Chemother. 1987 Apr;31(4):672–673. doi: 10.1128/aac.31.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson J. H., McCutchan T. F., Sogin M. L. Sequence of the small subunit ribosomal RNA gene expressed in the bloodstream stages of Plasmodium berghei: evolutionary implications. J Protozool. 1986 Nov;33(4):525–529. doi: 10.1111/j.1550-7408.1986.tb05656.x. [DOI] [PubMed] [Google Scholar]
- Gutell R. R., Weiser B., Woese C. R., Noller H. F. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1985;32:155–216. doi: 10.1016/s0079-6603(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Change in methylation of 16S ribosomal RNA associated with mutation to kasugamycin resistance in Escherichia coli. Nat New Biol. 1971 Sep 1;233(35):12–14. doi: 10.1038/newbio233012a0. [DOI] [PubMed] [Google Scholar]
- Jokipii L., Jokipii A. M. In vitro susceptibility of Giardia lamblia trophozoites to metronidazole and tinidazole. J Infect Dis. 1980 Mar;141(3):317–325. doi: 10.1093/infdis/141.3.317. [DOI] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Li M., Tzagoloff A., Underbrink-Lyon K., Martin N. C. Identification of the paromomycin-resistance mutation in the 15 S rRNA gene of yeast mitochondria. J Biol Chem. 1982 May 25;257(10):5921–5928. [PubMed] [Google Scholar]
- Lindley T. A., Chakraborty P. R., Edlind T. D. Heat shock and stress response in Giardia lamblia. Mol Biochem Parasitol. 1988 Mar;28(2):135–143. doi: 10.1016/0166-6851(88)90061-8. [DOI] [PubMed] [Google Scholar]
- Mata L. Cryptosporidium and other protozoa in diarrheal disease in less developed countries. Pediatr Infect Dis. 1986 Jan-Feb;5(1 Suppl):S117–S130. doi: 10.1097/00006454-198601001-00020. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Smith P. D., Gillin F. D., Kaushal N. A., Nash T. E. Antigenic analysis of Giardia lamblia from Afghanistan, Puerto Rico, Ecuador, and Oregon. Infect Immun. 1982 May;36(2):714–719. doi: 10.1128/iai.36.2.714-719.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler E. A., Blackburn E. H. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J Biol Chem. 1985 May 25;260(10):6334–6340. [PubMed] [Google Scholar]
- Vossbrinck C. R., Maddox J. V., Friedman S., Debrunner-Vossbrinck B. A., Woese C. R. Ribosomal RNA sequence suggests microsporidia are extremely ancient eukaryotes. 1987 Mar 26-Apr 1Nature. 326(6111):411–414. doi: 10.1038/326411a0. [DOI] [PubMed] [Google Scholar]


