Abstract
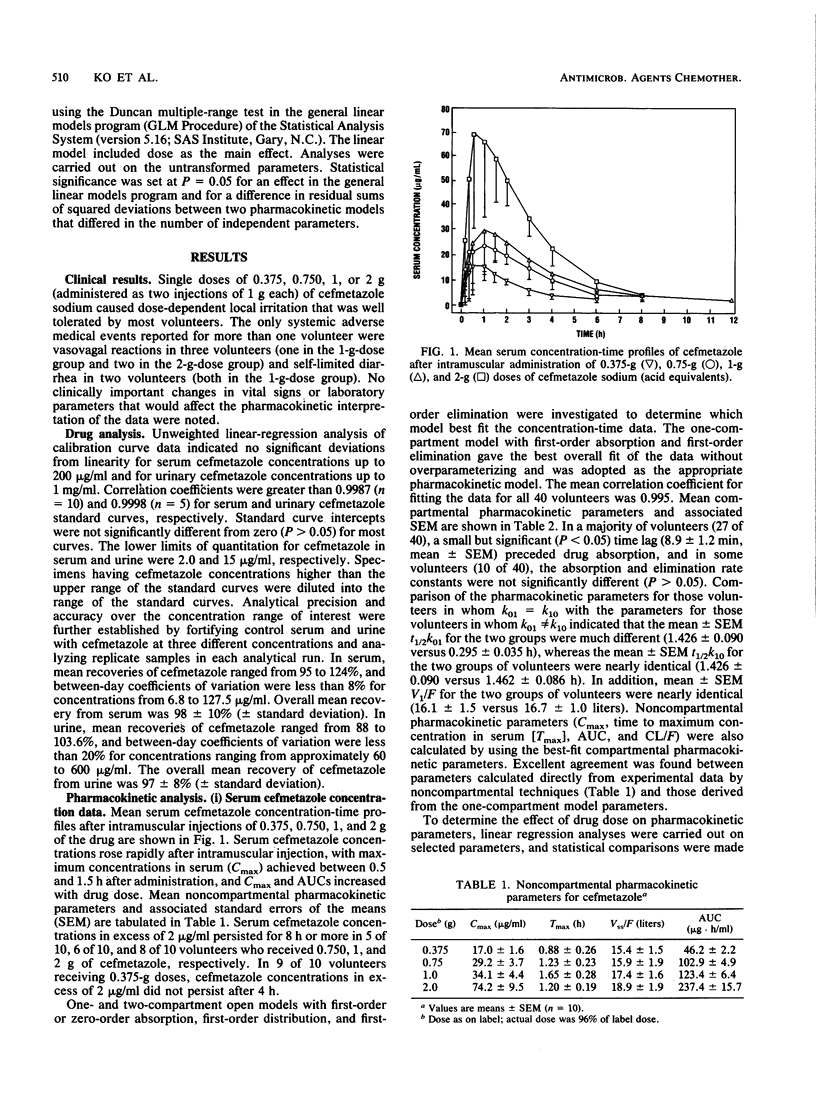
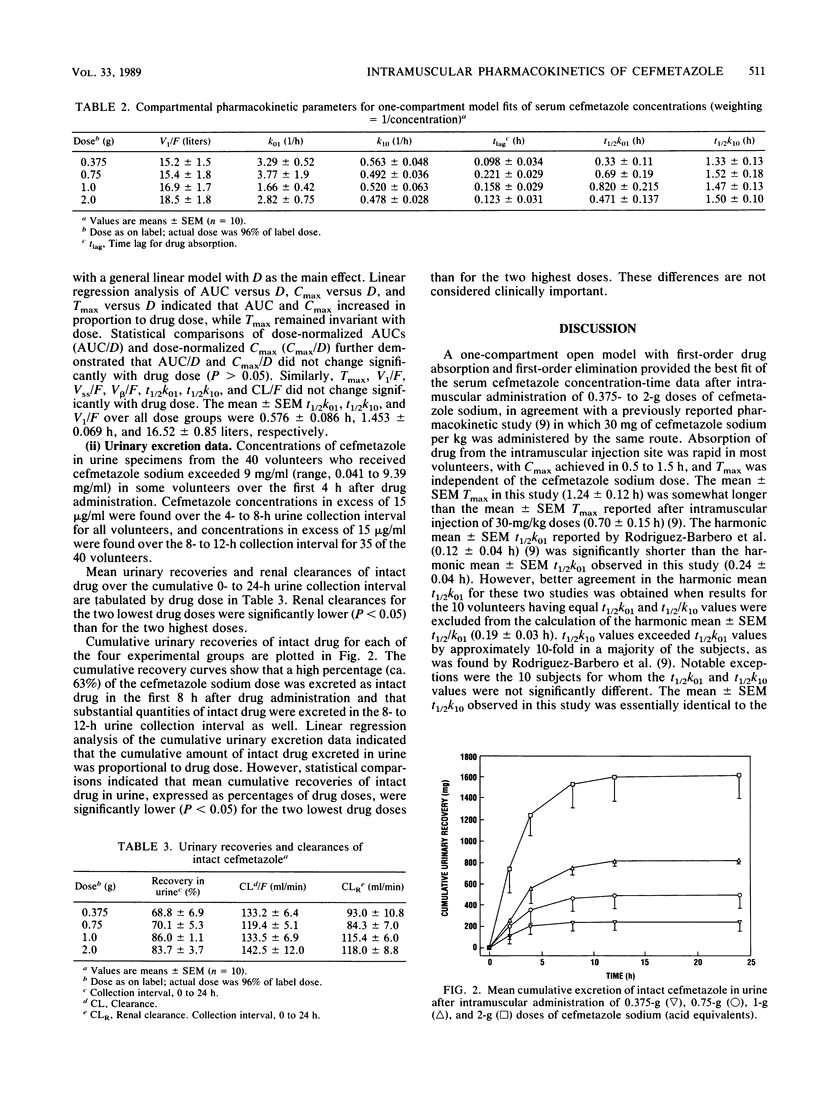
The tolerance and pharmacokinetics of cefmetazole were studied in healthy male volunteers who received a placebo (sterile saline) or a single dose of cefmetazole sodium intramuscularly. Drug-treated volunteers received one of four doses, 0.375, 0.750, 1, or 2 g. The drug was well tolerated, with no adverse medical events or laboratory changes observed during the study that could affect the pharmacokinetic interpretation of the data. Cefmetazole concentrations were determined by using a specific high-performance liquid chromatographic method. Serum cefmetazole concentrations were well described by a one-compartment open model with first-order absorption and elimination. Cefmetazole was rapidly absorbed in most volunteers, with a mean time to maximum concentration in serum of 1.24 +/- 0.12 h (+/- standard error of the mean), and the mean maximum concentration in serum increased from 17.0 +/- 1.6 to 74.2 +/- 9.5 micrograms/ml over the 0.375- to 2-g dose range. Maximum concentrations in serum, areas under serum concentration-time curve, and urinary excretion of intact drug increased in proportion to cefmetazole sodium dose. Times at which maximum concentrations in serum occurred, apparent volumes of distribution, steady-state volumes of distribution, absorption and elimination half-lives, and systemic clearances did not change significantly (P greater than 0.05) with drug dose. Although absorption and elimination half-lives were not significantly different in 10 of 40 volunteers (P greater than 0.05), in a majority of subjects elimination half-lives were approximately 10 times longer than absorption half-lives. The mean recovery of intact drug in urine ranged from 68.8 to 86.0% over the dose range studied, with a mean recovery over all doses of 77.1 +/- 2.4%. Rental clearances were significantly lower (P < 0.05) for the two lowest doses (93.0 and 84.3 versus 115.0 and 118.0 ml/min); these differences are not considered clinically important. The results of this study indicate that cefmetazole pharmacokinetics are linear after administration of single intramuscular doses ranging from 0.375 to 2 g, that clinically relevant concentrations of cefmetazole in serum (1 to 2 micrograms/ml) persist in a majority of volunteers for more than 8 h after administration of 0.750-g or higher doses, and that clinically relevant concentrations of cefmetazole continue to be excreted in urine 8 to 12 h after administration of 0.375- to 2-g doses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goto S., Sakamoto H., Ogawa M., Tsuji A., Kuwahara S. Bactericidal activity of cefazolin, cefoxitin, and cefmetazole against Escherichia coli and Klebsiella pneumoniae. Chemotherapy. 1982;28(1):18–25. doi: 10.1159/000238056. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Fuchs P. C., Thornsberry C. Antimicrobial activity of cefmetazole (CS-1170) and recommendations for susceptibility testing by disk diffusion, dilution, and anaerobic methods. J Clin Microbiol. 1986 Dec;24(6):1055–1059. doi: 10.1128/jcm.24.6.1055-1059.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H., Cathcart K. S., Griffith D. L., Peters G. R., Adams W. J. Pharmacokinetics of intravenously administered cefmetazole and cefoxitin and effects of probenecid on cefmetazole elimination. Antimicrob Agents Chemother. 1989 Mar;33(3):356–361. doi: 10.1128/aac.33.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao H., Yanagisawa H., Shimizu B., Kaneko M., Nagano M. A new semisynthetic 7alpha-methoxycephalosporin, CS-1170: 7beta-((cyanomethyl)thio)acetamido)-7alpha-methoxy-3-((1-methyl-1H-tetrazol-5-yl)thio)methyl)-3-cephem-4-carboxylic acid. J Antibiot (Tokyo) 1976 May;29(5):554–558. doi: 10.7164/antibiotics.29.554. [DOI] [PubMed] [Google Scholar]
- Ohkawa M., Orito M., Sugata T., Shimamura M., Sawaki M., Nakashita E., Kuroda K., Sasahara K. Pharmacokinetics of cefmetazole in normal subjects and in patients with impaired renal function. Antimicrob Agents Chemother. 1980 Sep;18(3):386–389. doi: 10.1128/aac.18.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier D., Mayersohn M. Noncompartmental determination of the steady-state volume of distribution for any mode of administration. J Pharm Sci. 1982 Mar;71(3):372–373. doi: 10.1002/jps.2600710332. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Barbero J., Mariño E. L., Dominguez-Gil A. Pharmacokinetics of cefmetazole administered intramuscularly and intravenously to healthy adults. Antimicrob Agents Chemother. 1985 Oct;28(4):544–547. doi: 10.1128/aac.28.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J. R., Mariño E. L., Otero M. J., Commes J. R., Garcia J., Rodriguez J. M., Lozano F., Dominguez-Gil A., Alonso A. G. Concentrations of cefmetazole in plasma and tissue resulting from peri-incisional administration before appendectomy. Antimicrob Agents Chemother. 1984 Nov;26(5):787–788. doi: 10.1128/aac.26.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrogie J. J., Davies R. O., Yeh K. C., Rogers D., Holmes G. I., Skeggs H., Martin C. M. Bioavailability and pharmacokinetics of cefoxitin sodium. J Antimicrob Chemother. 1978 Jul;4(B):69–78. doi: 10.1093/jac/4.suppl_b.69. [DOI] [PubMed] [Google Scholar]


