Abstract
The molecular mechanism used by environmental chemicals to exert their hormone-like actions is still only partially resolved. Although it generally is accepted that xenoestrogens act at the genomic level by binding to intracellular estrogen receptors, we have shown here that they trigger nongenomic effects in pancreatic β cells. Both xenoestrogens and the circulating hormone, 17β-estradiol, bind with high affinity to a common membrane binding site unrelated to the intracellular estrogen receptors ERα and ERβ. This binding site is shared by dopamine, epinephrine, and norepinephrine and has the pharmacological profile of the γadrenergic receptor. This study provides an outline of the membrane receptor involved in rapid xenoestrogen actions.
Keywords: pancreatic β cells, confocal microscopy, intracellular calcium, cell signaling
Xenoestrogens are compounds that present estrogenic effects but whose chemical structure does not necessarily resemble that of steroid hormones. They are found in fresh water and are likely responsible for the feminization of male fish in several rivers of the United Kingdom and the decreased reproduction success of alligators and turtles in Lake Apopka in Florida (1, 2). These manmade chemicals enter the body by ingestion or adsorption and mimic the genomic actions of estrogens (3) via intracellular estrogen receptors (4–6).
Less attention has been paid to nongenomic actions of xenoestrogens. Several studies have described that xenoestrogens mimic 17β-estradiol when they act on Ca2+ and K+ channels in smooth muscle cells (7) as well as when they trigger prolactin release from rat pituitary tumor cells (8). Yet, a direct link between an estrogen membrane binding site (9) and xenoestrogen actions is still a matter of debate. Moreover, despite the great number of studies about nongenomic actions of estrogens, a clear outline of the membrane receptor involved is still elusive.
We have chosen three widely used xenoestrogens for our study: bisphenol-A (BPA), which is found in the content of canned food, dental sealants, and composites; diethylstilbestrol (DES), a synthetic estrogen used between the 1940s and the 1970s to prevent miscarriages; and the well-known pesticide 1,1,1-trichloro-2-[o-chlorophenyl]-2-[p-chlorophenyl]ethane (o.p′-DDT) (3, 10–12). We have focused our research on the effect of these agents on the signaling system of pancreatic β cells, which are fundamental for the endocrine pancreas function. β cells, which are situated within the islet of Langerhans, are responsible for insulin secretion after an increase of blood glucose. A dysfunction of this particular type of cells causes the widespread pathology diabetes mellitus. An estrogen binding site at the plasma membrane of pancreatic β cells has been described (13). Once 17β-estradiol binds to this site, cGMP increases, activating protein kinase G, which phosphorylates ATP-dependent potassium channels (KATP), depolarizing the plasma membrane, and enhancing intracellular Ca2+ concentration ([Ca2+]i) signals. As a consequence, insulin secretion is increased (13, 14).
Here, we show that acute nongenomic actions induced by 17β-estradiol and xenoestrogens in pancreatic β cells occur after these substances are bound at a common membrane binding site. This is unrelated to the cytosolic/nuclear estrogen receptors ERα and ERβ and has the pharmacological profile of the so-called “γ-adrenergic receptor.”
Methods
Measuring Intracellular Calcium in β Cells Within Intact Islets of Langerhans.
Swiss albino OF1 male mice were killed by cervical dislocation. Pancreatic islets of Langerhans were isolated from mice by using collagenase as described (15). After isolation islets were loaded by incubation in 5 μM Fluo-3 AM (Molecular Probes) for at least 1 h at room temperature before imaging intracellular calcium. Calcium records in individual cells were obtained by imaging intracellular calcium under a Zeiss LSM 510 confocal microscope with a Zeiss ×40 oil immersion lens, numerical aperture 1.3. Images were collected at 2-s intervals, and fluorescence signals from individual cells were measured as a function of time by using the Zeiss lsm 510 software package. Experiments were performed at 34°C. Results were plotted by using commercially available software (sigmaplot, Jandel Scientific, San Rafael, CA) as the change in fluorescence intensity (ΔF) expressed as a percentage of the basal fluorescence intensity (F0) observed in the absence of stimulus. β cells within the islets were identified by their [Ca2+]i pattern in 3 mM and 8 mM glucose (16, 17).
Assay for Estradiol-Peroxidase Binding.
Islets were dispersed into single cells and cultured as described (18). Cells cultured on polylysine-coated coverslips for 24 h were fixed in 4% (wt/vol) paraformaldehyde for 30 s and exposed overnight to 4.5 μg/ml estradiol-peroxidase (Sigma) at 4°C. Cells then were washed, and estradiol-peroxidase conjugate [E-horseradish peroxidase (HRP)] binding was developed by using 0.5 mg/ml 3,3′-diaminobenzidine (DAB) tetrahydrochloride in the presence of 0.3 mg/ml urea hydrogen peroxide in 0.05 M Tris buffer and 0.15 M NaCl for 30 min (Sigma FAST DAB tablet set). In experiments performed for Fig. 5, 0.2 mg/ml Cl2Co was added (Sigma FAST DAB tetrahydrochloride with metal enhancer tablet set) to produce the complex Co-DAB, which gives a higher contrast compared with DAB polymers generated in the absence of metal additives. To test that the plasma membrane was not permeabilized, cells were incubated with 2.5 mg/ml dextran-conjugated tetramethylrhodamine (40,000 Da) and immediately visualized by using a Zeiss LSM 510 confocal microscope as described (13).
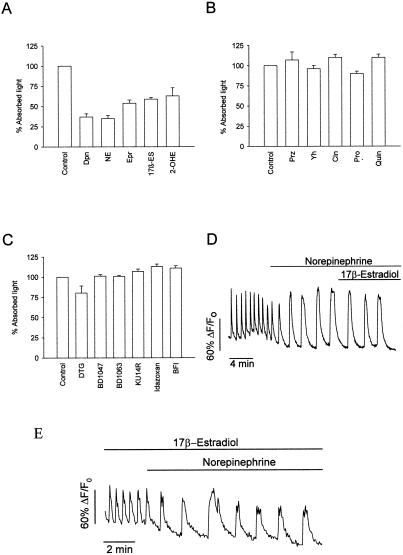
Figure 5.
General properties of the plasma membrane estrogen receptor. (A) Competition of E-HRP binding by the catecholamines dopamine (Dpn), NE, and epinephrine (E) and the catecholestrogen 2-hydroxyestradiol. These catechol-derived chemicals competed for the E-HRP binding as much as 17β-estradiol itself or even more as was the case of dopamine and NE. All of these chemicals were from Sigma. (B) Competition of E-HRP binding by the α1-adrenergic ligand prazosin (Prz), α2-adrenergic ligands yohimbine (Yh) and clonidine (Cln), β-adrenergic ligand propranolol (Pro), and the dopamine D2 receptor ligand quinpirole (Quin). Chemicals were from Tocris Neuramin, Bristol, U.K. (C) Absence of effect on the E-HRP binding of the σ-receptor ligands 1,3-Di(2-tolyl) guanidine (DTG), BD1047, and BD1063 and the imidazoline binding site ligands KU14R, Idazoxan, and 2-BFI. All of the substances were used at 30 μM concentration, but E-HRP was used at 100 nM. DMSO was used as a vehicle for liposoluble chemicals and had no effect on the E-HRP binding at the concentrations used. Results are representative of at least two different triplicate experiments. Chemicals were from Tocris. (D) Change in fluorescence intensity of Fluo-3 in single pancreatic β cells within intact islets of Langerhans. Islets were perfused with 8 mM glucose, and 100 nM NE was applied as specified by the bar. Once a stable [Ca2+]i pattern was established, 100 nM 17β-estradiol was added, producing no change in the frequency of the [Ca2+]i oscillations (0.7 ± 0.2 min-1 NE, 0.9 ± 0.3 NE + 17β- estradiol, n = 3 islets). When dopamine was used instead of NE, essentially the same results were obtained (0.8 ± 0.1 min-1 dopamine; 0.6 ± 0.2 dopamine + 17β-estradiol, n = 8 islets). (E) Change in fluorescence intensity of Fluo-3 in single pancreatic β cells within intact islets of Langerhans. Islets were perfused with 8 mM glucose, and 100 nM 17β-estradiol was applied. Once a stable [Ca2+]i pattern was established, 100 nM NE was applied. The change in the frequency of oscillations was from 2.2 ± 0.2 min-1 in the presence of estradiol to 0.67 ± 0.04 min-1 with NE (n = 3 islets). The same result was essentially obtained when using 10 μM 17β-estradiol. In control experiments the frequency was 1.3 ± 0.1 min-1 with 8 mM glucose only and decreased to 0.57 ± 0.11 with NE (n = 7 islets).
Because 65–80% of the islet cells are β cells, 15% are α cells, which can be easily identified by their particular morphology (17), we conclude that between 80% and 95% of the cells we used should be β cells. Nonetheless, we may overestimate the number of β cells by 5–20%.
Binding Studies Using E-HRP.
DAB-based primary reaction products of peroxidase are yellow-brown (DAB) or dark blue (Co-DAB complex); these compounds are highly absorptive for laser light at 488 nm. The absorbed light was used as an indication of the amount of E-HRP bound to the membrane, therefore it is possible to numerically assess binding of E-HRP by analyzing the light absorbed by the polymer formed by DAB or Co-DAB. Nonetheless, quantifying the amount of precipitate simply by measuring the light absorbed may be an unreliable procedure if differences of absorbed light are small. This method is of interest for comparative purposes, and it works extremely well for conditions in which the difference in the amount of precipitate is large. For that reason detailed concentration-response profiles have been omitted. Absorbed light was measured by taking transmission photographs with a Zeiss LSM 510 laser scanning confocal microscope, and the intensity of light along a line through the cell was quantified (see Fig. 2) by using the Zeiss lsm 510 software. Background light and microscope settings were constant throughout different experiments. To obtain an appropriate staining background, HRP was not conjugated to estradiol. When incubation and developmental procedure with HRP were performed in identical condition of experiments, a background absorbed light of 29 ± 7% (n = 150 cells from three different coverslips) was obtained (see Fig. 2D). This background light has not been subtracted.
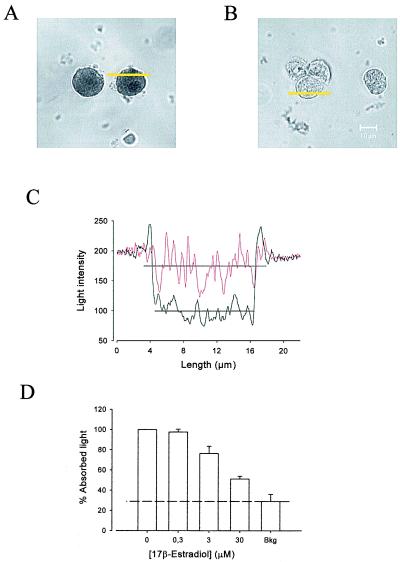
Figure 2.
E-HRP binding assay. (A) Binding of E-HRP (100 nM) to nonpermeabilized pancreatic islet cells in primary culture visualized by transmission (LSM) laser scanning. (B) Binding of E-HRP is blocked by using 300-fold excess 17β-estradiol. (C) Change in the intensity of light versus length along yellow lines in A. Intensity was measured on an arbitrary gray scale from 0 (blackest) to 255 (whitest). The black line corresponds to the control (cell in A) and the red line to the estradiol-blocked cell (cell in B). The horizontal black lines are the mean of the light intensity values within the cell, which are used as a measurement of the absorbed intensity of light in each condition. (D) Competition of E-HRP binding by increasing concentrations of 17β-estradiol. The quantity of E-HRP bound is expressed as the percentage of absorbed light with respect to the control condition. The lower the percentage of absorbed light the higher the competition for an E-HRP binding site. The control contained the same concentration of DMSO used for experiments. The results are expressed as the mean ± SEM of at least three different experiments with duplicate or triplicate samples. Bkg is the background absorbed light obtained by using HPR instead of E-HPR (see Methods). At least 50–100 single cells were counted per coverslip.
Immunocytochemistry.
Anti-ERα mAbs H222 and H226 and the affinity-purified peptide antibody ER21 were a kind gift of Geoffrey Greene, University of Chicago (19–22). Anti-ERα rabbit polyclonal antibodies G-20 and MC-20 and anti-ERβ goat polyclonal antibodies L-20 and Y-19 were from Santa Cruz Biotechnology. Cells cultured on polylysine-coated coverslips for 24 h were fixed in 4% (wt/vol) paraformaldehyde for 30 s and then incubated 30 min with 1% albumin supplemented with 2% fetal goat serum or normal donkey serum. After rinsing with PBS, cells were incubated with the corresponding antibodies (1:100 dilution) for 2 h at room temperature. After washing, FITC-conjugated secondary antibodies anti-mouse/anti-rabbit/anti-goat (1:100 dilution) were applied for 1 h at room temperature to visualize staining. To visualize intracellular labeling, cells were permeabilized after fixation with 1% Triton X-100 for 2 min; the rest of the protocol was followed as above. Confocal scanning laser microscopy was performed with a Zeiss LSM 510 microscope. The specificity of the labeling techniques was proven by the absence of labeling when either the first or the second antibodies were omitted.
Impeded Ligand Binding Assay.
Once cells were fixed as described above, they were incubated with antibodies G20, H222, Y19, and L20 for 2 h at 4°C. After that, an E-HRP binding assay was performed.
Results
Responses of β Cell [Ca2+]i to Xenoestrogens.
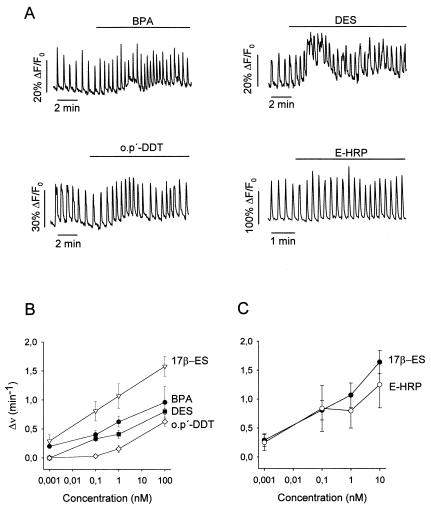
To investigate the role of xenoestrogens in pancreatic β cells, [Ca2+]i recordings were obtained from β cells within intact islets of Langerhans from male mice, using laser scanning confocal microscopy. When 1 nM of BPA and DES was applied in the presence of 8 mM glucose, an increase in the frequency of glucose-induced [Ca2+]i oscillations was observed (Fig. 1A). Both BPA and DES mimicked the effect of 17β-estradiol on [Ca2+]i signaling within a similar concentration range (Fig. 1B). In contrast, the effect of o.p′-DDT was milder, and alterations of the [Ca2+]i signals were not triggered until 100 nM o.p′-DDT was applied (Fig. 1B). These xenoestrogens, as well as 17β-estradiol (13), were without effect in the absence of glucose. These acute effects should be nongenomic because they were triggered in less than 3 min and were reproduced by the membrane-impermeable E-HRP (Fig. 1 A and C). The latter will be used as a probe to study the properties of the estradiol binding site (see below). The potentiation of [Ca2+]i signals by xenoestrogens is expected to produce an enhanced insulin secretion as demonstrated previously for 17β-estradiol (13).
Figure 1.
Xenoestrogens mimic 17β-estradiol in modulating [Ca2+]i. (A) Change in fluorescence intensity of Fluo-3 in single pancreatic β cells within intact islets of Langerhans. Islets were perfused with 8 mM glucose, and 1 nM BPA (Sigma), DES (Sigma), o.p′-DDT (Chem Service, Chester, Pa), and E-HRP (Sigma) were applied during the bar. The effects were not reversible after 20 min. Records are representative of at least five different experiments. (B) Change in the frequency of [Ca2+]i oscillations as a function of the concentration of 17β-estradiol (17β-ES), BPA, DES, and o.p′-DDT. Each point is the mean of at least five different experiments. The analysis of the frequency was done during 2- to 4-min periods, always taken when a steady state was reached, usually 4–8 min after stimuli application. Error bars shown are mean ± SEM. (C) Change in the frequency of [Ca2+]i oscillations as a function of the concentration of 17β-estradiol and E-HPR. Each point is the mean of at least three different experiments. The analysis of the frequency was done as described above.
A Common Membrane Binding Site for Xenoestrogens and 17β-Estradiol.
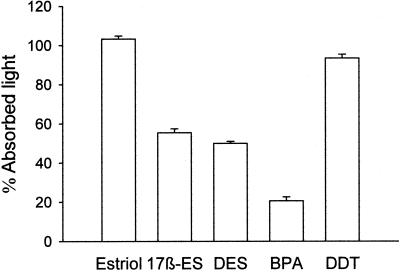
The mimicry found between BPA and DES with 17β-estradiol led us to examine whether xenoestrogens act by interacting with the same membrane binding site as the endogenous hormone. We have used a binding assay method based on binding of estradiol conjugated with HRP (E-HRP) as a specific probe to detect estradiol binding sites (13). The DAB-based primary reaction product of peroxidase (DAB or Co-DAB complex) was used as an indication of the amount of E-HRP bound to the estrogen binding site at the membrane of isolated nonpermeabilized cells. This primary reaction product is highly absorptive for laser light at 488 nm and can be easily visualized with high contrast by using transmission laser scanning microscopy (23). Fig. 2A shows two cells stained with E-HRP and developed by using DAB. The binding of E-HRP was blocked by competition with a 300-fold excess of 17β-estradiol as shown in Fig. 2B. A comparative numerical assessment of absorption intensities in single cells can be made by measuring the absorbed light in each desired condition (Fig. 2C). The quantity of E-HRP bound is expressed as the percentage of absorbed light with respect to the control condition. The lower the percentage of absorbed light the higher the competition for an E-HRP binding site. Fig. 2D shows how E-HRP binding was blocked by competition with 17β-estradiol in a dose-dependent manner. By using the described methodology, we were able to demonstrate that BPA, DES, and 17β-estradiol all compete for the same E-HRP binding site (Fig. 3). The pesticide o.p′-DDT, which was weaker in inducing [Ca2+]i signals, presented low competition. However, we should note that 100 nM o.p′-DDT modified the frequency of [Ca2+]i. This finding may indicate the presence of an alternative pathway used for other xenoestrogens with a different chemical structure than BPA and DES. Estriol, which did not present estrogenic activity on the [Ca2+]i signal (13), was used as a negative control with no effect on E-HRP binding (Fig. 3).
Figure 3.
Competition of E-HRP binding by estriol, 17β-estradiol, DES, BPA, and o.p′-DDT, all used at 30 μM. The results are expressed as the mean ± SEM of at least four different experiments with duplicate or triplicate samples.
The Membrane Estrogen Receptor Is Unrelated to ERα and ERβ.
The results presented up to now highly suggest that a common signal-generating receptor exists for 17β-estradiol and xenoestrogens on the cell surface of pancreatic β cells. Several reports have described the existence of proteins in the plasma membrane that cross-react with antibodies raised against cytosolic classical estrogen receptor (24, 25). These estrogen binding membrane proteins are functional in producing prolactin release from pituitary tumor cells (26).
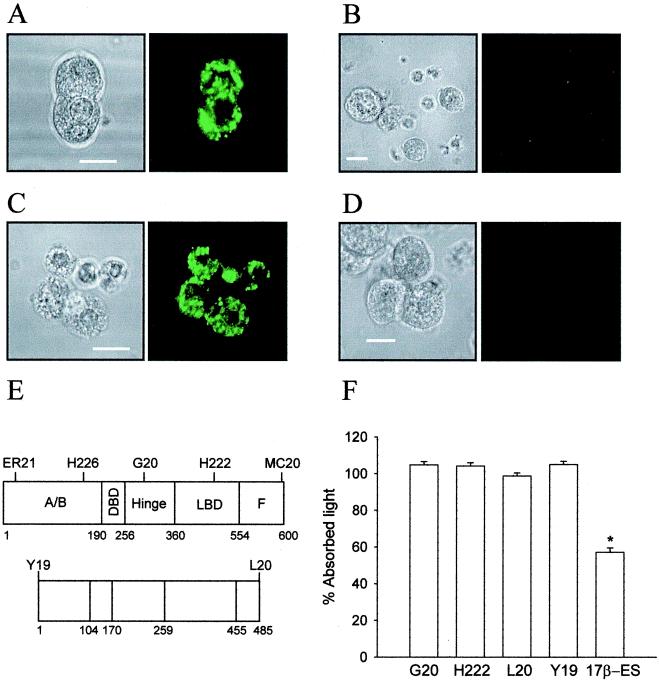
We have studied the possible structural similarity between the membrane estrogen binding site found in β cells and the intracellular estrogen receptors ERα and ERβ. Immunocytochemistry was performed, using five different antibodies raised against ERα and two anti-ERβ antibodies (Fig. 4E). It has been previously described that all of the used anti-ERα antibodies stain the plasma membrane of GH3/B6 rat pituitary tumor cell line and compete for an estradiol binding site (8, 24). Fig. 4A shows the staining of the cytoplasm with the H222 antibody, raised against the ligand binding domain of ERα, yet the plasma membrane was completely unstained (Fig. 4B). The same result was obtained with all of the anti-ERα antibodies described in Fig. 4E. To complete our study, we used two antibodies raised against the N terminus and the C terminus of ERβ. Neither one stained the membrane, yet both labeled the cytoplasm (Fig. 4 C and D). When the impeded ligand binding assay, using the antibodies G20 and H222 for ERα and Y19 and L20 for ERβ, was performed, none of them competed with E-HRP (Fig. 4F).
Figure 4.
(A) Anti-ERα antibody H222 stains the cytoplasm of permeabilized β cells in primary culture. (B) Absence of staining at the plasma membrane of nonpermeabilized β cells with anti-ERα antibody H222. These cells were from the same culture as the cells in A. (C) Anti-ERβ antibody Y19 stains the cytoplasm of permeabilized β cells in primary culture. (D) Lack of staining of Y19 antibody at the membrane of nonpermeabilized cells. These cells were from the same culture as those in C. Fluorescence pictures are on the right and their corresponding transmission pictures are on the left. Results are representative of three different duplicated experiments. (E) Epitopes of the intracellular ERα (upper map of the peptide) and ERβ (lower map of the peptide) by available anti-ERα and anti-ERβ antibodies. Epitopes are located by the antibodies named above. (F) Impeded binding ligand assay. E-HRP binding was not blocked either by anti-ERα antibodies H222 and G20 or by anti-ERβ antibodies Y19 and L20. Data are from three duplicated experiments, expressed as mean ± SEM, *, P < 10-5 Student's t test. (Scale bars are 10 μm.)
We conclude that the membrane estrogen receptor presents a different structure to that of ERα and ERβ.
The Plasma Membrane Estrogen Receptor Is a Catecholaminergic Receptor.
There is evidence that indicates that 17β-estradiol and the catecholestrogen 2-hydroxyestradiol may act by binding at the catecholamine receptors. For instance, competition of these two estrogens for dopamine and norepinephrine (NE) receptor binding has been described in membranes prepared from the cerebral cortex of the rat (27). It has been suggested that the α2-adrenergic receptor mediates the effect of 2-hydroxyestradiol on insulin secretion (28).
To study the possible implication of a catecholamine receptor in the actions described in this paper, we performed a competition of 2-hidroxyestradiol, dopamine, NE, and epinephrine for E-HRP binding. Remarkably, the binding of E-HRP was blocked equally as well by the three catecholamines as by the catecholestrogen 2-hidroxyestradiol (Fig. 5A).
To establish the pharmacological profile of the membrane estrogen binding site described here and to compare it with that of adrenergic and dopaminergic receptors, the experiment in Fig. 5B was performed. The binding of E-HPR was unaffected by prazosin at concentrations higher than the one required to completely occupy α1-adrenergic receptors (29). Similar results were obtained with the α2-adrenergic receptor ligands, yohimbine and clonidine, with propranolol, a β-adrenergic receptor ligand, and with the D2 dopamine receptor antagonist quinpirole (Fig. 5B). Binding of E-HRP seems to be specifically blocked by compounds with catechol rings and those with the A ring of 17β-estradiol. Other lipophilic substances containing neither catechol nor A rings, such as sigma and imidazoline binding site ligands, had no effect (Fig. 5C).
The functional interaction between 17β-estradiol and catecholamines was studied by measuring the effect of 17β-estradiol on NE and dopamine-induced changes in [Ca2+]i. The application of 100 nM NE led to a modification of the [Ca2+]i pattern, mainly because of the activation of the α2-adrenergic receptor (30). When 17β-estradiol was applied in the presence of NE, it had no effect on the oscillatory pattern (Fig. 5D). When NE was applied in the presence of a high concentration of 17β-estradiol, which should saturate all estradiol binding sites, the NE response was unchanged (Fig. 5E).
Essentially the same results were obtained when dopamine was used instead of NE. Accordingly, NE and dopamine appear to antagonize the nongenomic action of 17β-estradiol on the [Ca2+]i signal, likely by binding at the same receptor. The fact that high concentrations of 17β-estradiol (100 nM and 10 μM) had no effect on the NE response indicates that this receptor should be different from the classic α and β adrenergic receptor.
Discussion
This report demonstrates that the circulating hormone 17β-estradiol and the xenoestrogens BPA and DES enhance the frequency of [Ca2+]i oscillations in pancreatic β cells within intact islets of Langerhans. The effect of these agents, although modest, is well within the range of other insulin secretion modulators such as vasoactive intestinal polipeptide (31), gastrin releasing peptide (32), and glucagon-like peptide-1 (33). Because insulin secretion mirrors the [Ca2+]i pattern (34, 35) a rise of insulin secretion would be expected, as previously described for 17β-estradiol (13). This nongenomic effect is triggered by remarkably low concentrations of BPA and DES, within the nanomolar range. The strong action of BPA is remarkable, because it has been shown to behave as a weaker estrogen, about 1,000- to 2,000-fold less potent than 17β-estradiol when interacting with the nuclear estrogen receptor (36, 37). This finding indicates an action via a receptor different to the nuclear estrogen receptor.
It has been suggested that estrogen receptors at the plasma membrane may be involved in the rapid nongenomic effects of estrogens in several types of cells (38, 39), including pancreatic β cells (13, 14) although their characterization remains unresolved. Several presumptions about the molecular structure of the estrogen receptor involved in acute actions of estrogens and xenoestrogens have been suggested. Evidence has been published showing that the membrane estrogen receptor has a similar structure to ERα (24, 25, 40). This receptor would be implicated in triggering nongenomic actions of xenoestrogens (8). In contrast, evidence against that hypothesis also has been reported (28, 41–43). We have demonstrated here the existence of a membrane estrogen receptor involved in the nongenomic actions of estrogens and xenoestrogens, unrelated to both ERα and ERβ.
The latter is also the case with a recently characterized putative progesterone membrane binding protein, which shows no significant identity to the classic intracellular receptor (44, 45). Likewise, testosterone acts via a plasma receptor, which differs from the intracellular androgen receptor (46). These data may indicate the existence of a membrane steroid receptor superfamily, which is different from that of intracellular steroid receptors.
This membrane estrogen receptor not only binds 17β-estradiol, BPA, and DES but also the neurotransmitters epinephrine, NE, and dopamine. An excitatory receptor equally activated by dopamine, epinephrine, and NE has been described in the vascular (47–49) and nervous systems (50, 51). This receptor is pharmacologically different from the α or β adrenergic receptor. It is affected neither by the α nor the β antagonist, and it has been defined as the γ-adrenergic receptor (47–49). These properties match those described for the membrane estrogen binding site in the present work.
Moreover, the pathway involved in γ-adrenergic receptor action is a nitric oxide-independent guanylyl cyclase, which increases cGMP, activating protein kinase G (51). This pathway exactly imitates the one used by 17β-estradiol for modulating [Ca2+]i signals and KATP channels in pancreatic β cells (14). The findings show that the so-called γ-adrenergic receptor behaves as a membrane estrogen receptor.
Our results demonstrate a functional membrane receptor activated by the endogenous hormone 17β-estradiol as well as by the xenoestrogens BPA and DES, providing knowledge about the molecular mechanism involved in the nongenomic action of xenoestrogens.
The data define the pharmacological properties of this type of membrane estrogen receptor and unveil a level of cross-talk between estrogens and biogenic catecholamines. The latter may constitute an important link between the endocrine and the nervous systems.
Acknowledgments
We thank E. Perez Garcia and A. Perez Vegara for technical assistance and Drs. Pedro Verdugo, Ivan Quesada, and Cristina Ripoll for their help in several aspects of the project. This work was supported by Grants from Ministerio de Educación y Cultura (Grants PM98/0105 and PM99/0142); European Union-Comision Interministerial de Ciencia y Tecnologia (IFD97–1065-103–02), and Fundació Marató TV3 (99–1210). A.B.R. is a recipient of a research scholarship from the Ministerio de Educación y Cultura, O.L. is a recipient of a scholarship from the Ministerio de Asuntos Exteriores, and M.M. was an ERASMUS/SOCRATES program student.
Abbreviations
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- DES
diethylstilbestrol
- BPA
bisphenol-A
- o.p′-DDT
1,1,1-trichloro-2-[o-chlorophenyl]-2-[p-chlorophenyl]ethane
- E-HRP
estradiol-horseradish peroxidase
- DAB
3,3′-diaminobenzidine
- NE
norepinephrine
- [Ca2+]i
intracellular Ca2+ concentration
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Guillette L J, Gross T S, Masson G R, Matter J M, Percival H F, Woodward A R. Environ Health Perspect. 1994;102:680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillette L J, Pickford D B, Crain D A, Rooney A A, Percival H F. Gen Comp Endocrinol. 1996;101:32–42. doi: 10.1006/gcen.1996.0005. [DOI] [PubMed] [Google Scholar]
- 3.Sonnenschein C, Soto A M. J Steroid Biochem Mol Biol. 1998;65:143–149. doi: 10.1016/s0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- 4.Mueller G, Kim U H. Endocrinology. 1978;102:1429–1435. doi: 10.1210/endo-102-5-1429. [DOI] [PubMed] [Google Scholar]
- 5.McLachlan J A. Environ Health Perspect. 1993;101:386–387. doi: 10.1289/ehp.93101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soto A M, Chung K L, Sonnenschein C. Environ Health Perspect. 1994;102:380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruehlmann D O, Steinert J R, Valverde M A, Jacob R, Mann G E. FASEB J. 1998;12:613–619. doi: 10.1096/fasebj.12.7.613. [DOI] [PubMed] [Google Scholar]
- 8.Watson C S, Campbell C H, Gametchu B. Exp Physiol. 1999;84:1013–1022. doi: 10.1111/j.1469-445x.1999.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pietras R J, Szego C M. Nature (London) 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 10.Brotons J A, Olea-Serrano M F, Villalobos M, Olea N. Environ Health Perspect. 1994;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinmetz R, Mitcher N A, Grant A, Allen D L, Bigsby R M, Ben-Jonathan N. Endocrinology. 1998;139:2741–2747. doi: 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- 12.Das S, Thomas P. Endocrinology. 1999;140:1953–1956. doi: 10.1210/endo.140.4.6781. [DOI] [PubMed] [Google Scholar]
- 13.Nadal A, Rovira J M, Laribi O, León-Quinto T, Andreu E, Ripoll C, Soria B. FASEB J. 1998;12:1341–1348. doi: 10.1096/fasebj.12.13.1341. [DOI] [PubMed] [Google Scholar]
- 14.Ropero A B, Fuentes E, Rovira J M, Ripoll C, Soria B, Nadal A. J Physiol (London) 1999;521:397–407. doi: 10.1111/j.1469-7793.1999.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadal A, Valdeolmillos M, Soria B. Am J Physiol. 1994;267:E769–E774. doi: 10.1152/ajpendo.1994.267.5.E769. [DOI] [PubMed] [Google Scholar]
- 16.Nadal A, Quesada I, Soria B. J Physiol (London) 1999;517:85–93. doi: 10.1111/j.1469-7793.1999.0085z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quesada I, Nadal A, Soria B. Diabetes. 1999;48:2390–2397. doi: 10.2337/diabetes.48.12.2390. [DOI] [PubMed] [Google Scholar]
- 18.Valdeolmillos M, Nadal A, Contreras D, Soria B. J Physiol (London) 1992;455:173–186. doi: 10.1113/jphysiol.1992.sp019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene G L, Sobel N B, King W J, Jensen E V. J Steroid Biochem. 1984;20:51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- 20.Kumar V, Green G L, Staub A, Chambon P. EMBO J. 1986;5:2231–2236. doi: 10.1002/j.1460-2075.1986.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blaustein J D. Endocrinology. 1993;132:1218–1224. doi: 10.1210/endo.132.3.7679973. [DOI] [PubMed] [Google Scholar]
- 22.Hess R A, Gist D H, Bunick D, Lubahn D B, Farrell A, Bahr J, Cooke P S, Greene G L. J Androl. 1997;18:602–611. [PubMed] [Google Scholar]
- 23.Halbhuber K J, Krier R, König K. Cell Mol Biol. 1998;44:807–826. [PubMed] [Google Scholar]
- 24.Pappas T C, Gametchu B, Watson C S. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 25.Razandi M, Pedram A, Greene G L, Levin E R. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 26.Norfleet A M, Clarke C H, Gametchu B, Watson C S. FASEB J. 2000;14:157–165. doi: 10.1096/fasebj.14.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paden C M, McEwen B S, Fishman J, Snider L, DeGroff V. J Neurochem. 1982;39:512–520. doi: 10.1111/j.1471-4159.1982.tb03974.x. [DOI] [PubMed] [Google Scholar]
- 28.Etchegoyen G S, Borelli M I, Rossi J P F C, Gagliardino J J. Diabetes Metab. 1998;24:428–433. [PubMed] [Google Scholar]
- 29.Davey M J. Med J Aust. 1980;2:4–8. [PubMed] [Google Scholar]
- 30.Chan S L. Clin Sci. 1993;85:671–677. doi: 10.1042/cs0850671. [DOI] [PubMed] [Google Scholar]
- 31.Jensen S L, Fahrenkrug J, Holst J J, Vang Nielsen O, Schaffalizky de Muckadell O B. Am J Physiol. 1978;235:E387–E391. doi: 10.1152/ajpendo.1978.235.4.E387. [DOI] [PubMed] [Google Scholar]
- 32.Wahl M A, Plehn R J, Landsbeck E A, Verspohl E J, Ammon H P T. Endocrinology. 1991;128:3247–3252. doi: 10.1210/endo-128-6-3247. [DOI] [PubMed] [Google Scholar]
- 33.Holz G G, Kúhtreiber W H, Habener J F. Nature (London) 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin F, Sánchez-Andrés J V, Soria B. Diabetes. 1995;44:300–305. doi: 10.2337/diab.44.3.300. [DOI] [PubMed] [Google Scholar]
- 35.Barbosa R M, Silva A M, Tome A R, Stamford J A, Santos R M, Rosario L M. Biochem Biophys Res Commun. 1996;228:100–104. doi: 10.1006/bbrc.1996.1622. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan A V, Stathis P, Permuth S F, Tokes L, Feldman D. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 37.Kuiper G G M J, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 38.Wehling M. Annu Rev Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- 39.Alonso R, López-Coviella I. Neurochem Res. 1998;23:679–692. doi: 10.1023/a:1022442922931. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Yuhanna I S, Galcheva-Gargova Z, Karas R H, Mendelsohn M E, Shaul P W. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng J B, Ramirez V D. J Steroids Biochem Mol Biol. 1999;68:65–75. doi: 10.1016/s0960-0760(98)00161-7. [DOI] [PubMed] [Google Scholar]
- 42.Gu Q, Korach K S, Moss R L. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- 43.Valverde M A, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde M I, Mann G E, Vergara C, Latorre R. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 44.Gerdes D, Wehling M, Leube B, Falkenstein E. Biol Chem. 1998;379:907–911. doi: 10.1515/bchm.1998.379.7.907. [DOI] [PubMed] [Google Scholar]
- 45.Falkenstein E, Heck M, Gerdes D, Grube D, Christ M, Weigel M, Buddhikot M, Meizel S, Wehling M. Endocrinology. 1999;140:5999–6002. doi: 10.1210/endo.140.12.7304. [DOI] [PubMed] [Google Scholar]
- 46.Benten W P M, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F. Mol Biol Cell. 1999;10:3113–3123. doi: 10.1091/mbc.10.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirst G D S, Neild T O. Nature (London) 1980;283:767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- 48.Hirst G D S, Neild T O, Silverberg G D. J Physiol (London) 1982;328:351–360. doi: 10.1113/jphysiol.1982.sp014268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benham C D, Tsien R W. J Physiol (London) 1988;404:767–784. doi: 10.1113/jphysiol.1988.sp017318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yawo H. J Physiol (London) 1996;493:385–391. doi: 10.1113/jphysiol.1996.sp021390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yawo H. J Neurosci. 1999;19:5293–5300. doi: 10.1523/JNEUROSCI.19-13-05293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]