Abstract
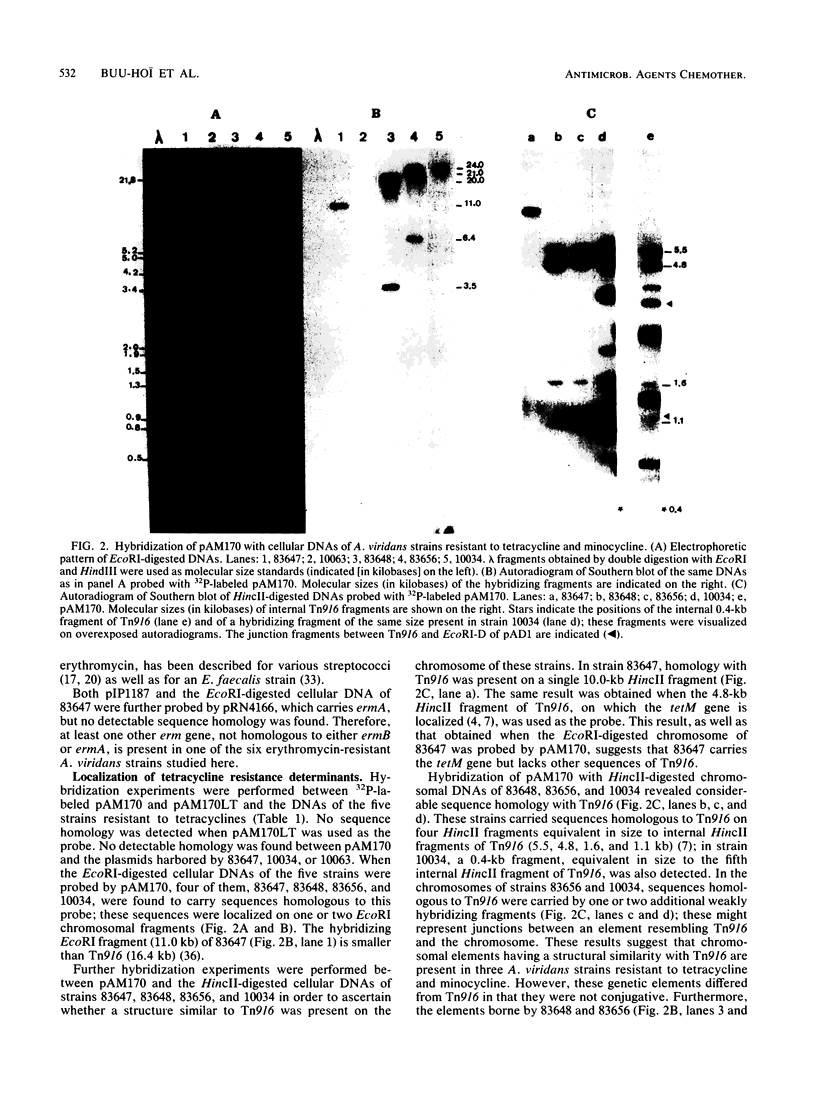
Resistance to at least one of the following antibiotics was found in eight wild-type strains of Aerococcus viridans: erythromycin (six strains), tetracycline and minocycline (five strains), chloramphenicol (one strain), and high levels of streptomycin (one strain). None of the strains transferred any of their antibiotic resistance markers into streptococcal, enterococcal, or A. viridans recipients by conjugation. By DNA-DNA hybridization experiments, the ermB gene of transposon Tn917, of Enterococcus faecalis origin, was detected in five of the six strains resistant to erythromycin and was localized for one strain on the chromosome and for four strains on nonconjugative small (4.7- to 4.9-kilobase) plasmids. The tetM gene of the conjugative transposon Tn916, of E. faecalis origin, was localized on the chromosome of four of the five strains resistant to tetracycline and minocycline; in three of these strains a structure similar to that of Tn916 was found. Homology to the tetO gene of pUA466, of Campylobacter jejuni origin, was detected on the chromosome of the fifth strain. No sequence homology was detected in any strain with probes corresponding to the tetL gene of group B Streptococcus origin, to the ermA gene of the transposon Tn554 of Staphylococcus aureus origin, or to the cat genes of either pC194 or pC221 of S. aureus origin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-Hoï A., Bieth G., Horaud T. Broad host range of streptococcal macrolide resistance plasmids. Antimicrob Agents Chemother. 1984 Feb;25(2):289–291. doi: 10.1128/aac.25.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-Hoï A., Branger C., Acar J. F. Vancomycin-resistant streptococci or Leuconostoc sp. Antimicrob Agents Chemother. 1985 Sep;28(3):458–460. doi: 10.1128/aac.28.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Tomich P. K., Gawron-Burke M. C., Franke A. E., Yagi Y., An F. Y. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J Bacteriol. 1982 Dec;152(3):1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. E., Clarke R. W., Maskell R. Streptococci as urinary pathogens. Lancet. 1986 Aug 30;2(8505):479–481. doi: 10.1016/s0140-6736(86)90356-9. [DOI] [PubMed] [Google Scholar]
- Colman G. Aerococcus-like organisms isolated from human infections. J Clin Pathol. 1967 May;20(3):294–297. doi: 10.1136/jcp.20.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. A transposon in Streptococcus faecalis with fertility properties. Nature. 1982 Nov 18;300(5889):281–284. doi: 10.1038/300281a0. [DOI] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bouanchaud D. H., Bieth G., Chabbert Y. A. R plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1976 Nov;10(5):795–801. doi: 10.1128/aac.10.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., Bieth G. Conjugative transfer of multiple-antibiotic resistance markers in beta-hemolytic group A, B, F, and G streptococci in the absence of extrachromosomal deoxyribonucleic acid. Plasmid. 1981 Mar;5(2):127–137. doi: 10.1016/0147-619x(81)90014-7. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bouanchaud D. H., Chabbert Y. A. Conjugative R plasmids in Streptococcus agalactiae (group B). Plasmid. 1979 Apr;2(2):197–206. doi: 10.1016/0147-619x(79)90038-6. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Buu-Hoï A., Delbos F., Bieth G. High-level aminoglycoside resistance in group A, B, G, D (Streptococcus bovis), and viridans streptococci. Antimicrob Agents Chemother. 1982 Jan;21(1):176–179. doi: 10.1128/aac.21.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Buu-Hoï A., Le Bouguenec C., Bieth G. Narrow host range of some streptococcal R plasmids. Plasmid. 1982 Sep;8(2):199–206. doi: 10.1016/0147-619x(82)90057-9. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janosek J., Eckert J., Hudác A. Aerococcus viridans as a causative agent of infectious endocarditis. J Hyg Epidemiol Microbiol Immunol. 1980;24(1):92–96. [PubMed] [Google Scholar]
- Kerbaugh M. A., Evans J. B. Aerococcus viridans in the hospital environment. Appl Microbiol. 1968 Mar;16(3):519–523. doi: 10.1128/am.16.3.519-523.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouguenec C., Horaud T., Bieth G., Colimon R., Dauguet C. Translocation of antibiotic resistance markers of a plasmid-free Streptococcus pyogenes (group A) strain into different streptococcal hemolysin plasmids. Mol Gen Genet. 1984;194(3):377–387. doi: 10.1007/BF00425548. [DOI] [PubMed] [Google Scholar]
- Le Bouguénec C., de Cespédès G., Horaud T. Molecular analysis of a composite chromosomal conjugative element (Tn3701) of Streptococcus pyogenes. J Bacteriol. 1988 Sep;170(9):3930–3936. doi: 10.1128/jb.170.9.3930-3936.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N., Titmas B. M., Smith C. J., Tenover F. C. Nucleotide sequence analysis of tetracycline resistance gene tetO from Streptococcus mutans DL5. J Bacteriol. 1988 Aug;170(8):3618–3626. doi: 10.1128/jb.170.8.3618-3626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. E., Jr Gram-positive bacteria: spread and antimicrobial resistance in university and community hospitals in the USA. J Antimicrob Chemother. 1988 Apr;21 (Suppl 100):49–55. doi: 10.1093/jac/21.suppl_c.49. [DOI] [PubMed] [Google Scholar]
- Murphy E., Phillips S., Edelman I., Novick R. P. Tn554: isolation and characterization of plasmid insertions. Plasmid. 1981 May;5(3):292–305. doi: 10.1016/0147-619x(81)90006-8. [DOI] [PubMed] [Google Scholar]
- Nathavitharana K. A., Arseculeratne S. N., Aponso H. A., Vijeratnam R., Jayasena L., Navaratnam C. Acute meningitis in early childhood caused by Aerococcus viridans. Br Med J (Clin Res Ed) 1983 Apr 16;286(6373):1248–1248. doi: 10.1136/bmj.286.6373.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Parker M. T., Ball L. C. Streptococci and aerococci associated with systemic infection in man. J Med Microbiol. 1976 Aug;9(3):275–302. doi: 10.1099/00222615-9-3-275. [DOI] [PubMed] [Google Scholar]
- Pepper K., Horaud T., Le Bouguénec C., de Cespédès G. Location of antibiotic resistance markers in clinical isolates of Enterococcus faecalis with similar antibiotypes. Antimicrob Agents Chemother. 1987 Sep;31(9):1394–1402. doi: 10.1128/aac.31.9.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper K., Le Bouguénec C., de Cespédès G., Horaud T. Dispersal of a plasmid-borne chloramphenicol resistance gene in streptococcal and enterococcal plasmids. Plasmid. 1986 Nov;16(3):195–203. doi: 10.1016/0147-619x(86)90057-0. [DOI] [PubMed] [Google Scholar]
- Pepper K., de Cespédès G., Horaud T. Heterogeneity of chromosomal genes encoding chloramphenicol resistance in streptococci. Plasmid. 1988 Jan;19(1):71–74. doi: 10.1016/0147-619x(88)90065-0. [DOI] [PubMed] [Google Scholar]
- Pien F. D., Wilson W. R., Kunz K., Washington J. A., 2nd Aerococcus viridans endocarditis. Mayo Clin Proc. 1984 Jan;59(1):47–48. doi: 10.1016/s0025-6196(12)60342-5. [DOI] [PubMed] [Google Scholar]
- Senghas E., Jones J. M., Yamamoto M., Gawron-Burke C., Clewell D. B. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol. 1988 Jan;170(1):245–249. doi: 10.1128/jb.170.1.245-249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Clewell D. B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985 Nov;164(2):782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Hiratsuka K., Ray H., Manavathu E. K. Characterization and expression of a cloned tetracycline resistance determinant from Campylobacter jejuni plasmid pUA466. J Bacteriol. 1987 Jul;169(7):2984–2989. doi: 10.1128/jb.169.7.2984-2989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untereker W. J., Hanna B. A. Endocarditis and osteomyelitis caused by Aerococcus viridans. Mt Sinai J Med. 1976 May-Jun;43(3):248–252. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. E., HIRCH A., COWAN S. T. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953 Jun;8(3):475–480. doi: 10.1099/00221287-8-3-475. [DOI] [PubMed] [Google Scholar]
- Wilson C. R., Skinner S. E., Shaw W. V. Analysis of two chloramphenicol resistance plasmids from Staphylococcus aureus: insertional inactivation of Cm resistance, mapping of restriction sites, and construction of cloning vehicles. Plasmid. 1981 May;5(3):245–258. doi: 10.1016/0147-619x(81)90002-0. [DOI] [PubMed] [Google Scholar]
- Yagi Y., McLellan T. S., Frez W. A., Clewell D. B. Characterization of a small plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain of Streptococcus sanguis isolated from dental plaque. Antimicrob Agents Chemother. 1978 May;13(5):884–887. doi: 10.1128/aac.13.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]